Metal-Organic Frameworks: Derived from Single Molecule Magnets.
Katsenis, A. D.; Brechin, E. K.; Papaefstathiou G. SAbstract
In this account, we discuss some metal–organic frameworks (MOFs) that are based on single‐molecule magnets (SMMs). Although the fields of MOFs and SMMs have independently grown rapidly over the last two decades, examples of SMM‐based MOFs are limited, with those possessing a 3D framework numbering just a handful. MOFs are robust porous molecular materials that are predominantly based on polynuclear metal complexes (clusters) owing their properties to their open framework structures. SMMs, on the other hand, are interesting in their own right as they constitute a very special kind of polynuclear metal complex, having the ability to store magnetic information at the molecular level. Although the properties of SMMs are of molecular origin, they can be influenced by crystal‐packing effects, especially at low temperatures. As expected, the combination of these two molecular materials benefits both fields, as SMMs can carry their magnetic properties to a MOF, while their incorporation within a framework may alter/modulate their magnetic properties. Therefore, a new class of molecular materials rises from the combination of the two. Although this new field is very much in its infancy, early examples of SMM‐based MOFs promise much exciting chemistry (and physics) for both academic intuition and an applications‐driven perspective. We hope that this account will inspire scientists to make many more such SMM‐based MOFs and study their properties.
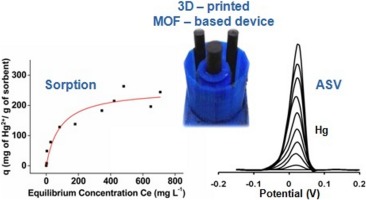
3D-printed lab-in-a-syringe voltammetric cell based on a working electrode modified with a highly efficient Ca-MOF sorbent for the determination of Hg(II).
Kokkinos, C., Economou, A., Pournara, A., Manos, M., Spanopoulos, I., Kanatzidis, M., Tziotzi, T., Petkov, V., Margariti, A., Oikonomopoulos, P. & Papaefstathiou, G. S.Abstract
This work combines, for the first time, 3D-printing technology and a highly efficient metal organic framework (Ca-MOF) as an electrode modifier to produce a novel fully integrated lab-in-a-syringe device for the sensitive determination of Hg(II) by anodic stripping voltammetry. The specific Ca-MOF ([Ca(H4L)(DMA)2]·2DMA where H6L is the N,N’-bis(2,4-dicarboxyphenyl)-oxalamide and DMA is the N,N-dimethylacetamide) shows an exceptional Hg(II) sorption capability over a wide pH range and its mechanism is elucidated via spectroscopic and X-ray diffraction studies. The voltammetric lab-in-a-syringe device is fabricated through a single-step process using a dual extruder 3D printer and is composed of a vessel integrating two thermoplastic conductive electrodes (serving as the counter and pseudo-reference electrodes) and of a small detachable 3D-printed syringe loaded with a graphite paste/Ca-MOF mixture (which serves as the working electrode). After optimization of the fabrication and operational variables, a limit of detection of 0.6 μg L−1 Hg(II) was achieved, which is comparable or lower than that of existing sensors (plastic 3D-printed, gold and MOF-based electrodes). The adoption of 3D printing technology in combination with the highly efficient Ca-MOF enables the fabrication of a simple, low-cost and sensitive electrochemical sensor for Hg(II), which is suitable for on-site applications.
Chemically modified electrodes with MOFs for the determination of inorganic and organic analytes via voltammetric techniques: a critical review.
Pournara A. D., Tarlas G. D., Papaefstathiou G. S., & Manos M. J.Abstract
Voltammetric analytical techniques combine exceptional sensitivity, low cost, portability and capability for simultaneous determination of multiple analytes. The sensitivity of voltammetric analysis is largely determined by the efficiency of the working electrode. Electrodes modified with metal organic frameworks (MOFs) seem particularly promising for use in the analysis of a series of important inorganic and organic analytes. Nevertheless, research on chemically modified electrodes with MOFs is still in its infancy. In this critical review, we present the current status of research related to MOF-modified electrodes highlighting the respective MOF-modified electrodes which are based on MOFs that show exceptional chemical stability or/and sorption capability towards the targeted analytes. We also provide perspectives for future research aiming at motivating additional scientists to be involved in this exciting field of MOF-based electroanalytical sensors.
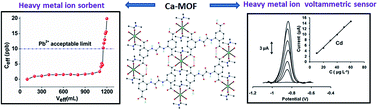
A Ca2+ MOF combining highly efficient sorption and capability for voltammetric determination of heavy metal ions in aqueous media.
Pournara A.D., Margariti A., Tarlas G.D., Kourtelaris A., Petkov V., Kokkinos C., Economou A., Papaefstathiou G.S., Manos M.J.Abstract
We recently discovered that a Ca2+ two-dimensional framework, namely [Ca(H4L)(DMA)2]·2DMA (Ca-MOF), was capable of exchanging the Ca2+ ions with Cu2+ almost quantitatively in a matter of seconds in aqueous solution. Herein, we report that Ca-MOF exhibits the capability of both removal and voltammetric determination of heavy metal ions in aqueous media. Ca-MOF shows one of the highest Pb2+ sorption capacities (∼522 mg g−1) reported for MOFs. More importantly, a column filled with Ca-MOF (1% wt) particles dispersed in silica sand (99% wt) can quantitatively remove traces of Pb2+ (∼100 ppb) from a relatively large volume of a wastewater simulant solution (containing a large excess of competitive ions). Ca-MOF is also highly efficient for sorption of Cd2+, Ni2+ and Zn2+, even in the presence of several competitive cations. Actually, the Cd2+ sorption capacity (∼220 mg g−1) of Ca-MOF is one of the largest reported for MOFs. Furthermore, detailed Ni2+ and Zn2+ sorption studies of MOFs have not been described prior to this work. The mechanism of the M2+ (M2+ = Pb2+, Cd2+, Ni2+, Zn2+) exchange process was elucidated based on a series of spectroscopic, analytical and X-ray diffraction methods. In addition, a simple ready-to-use electrochemical sensor based on modified graphite paste with Ca-MOF was fabricated and successfully utilized for the determination of Pb2+, Cd2+, Cu2+ and Zn2+ at μg L−1 levels in aqueous solutions by anodic stripping voltammetry (ASV). Overall, this work demonstrates, for the first time, a dual function of a MOF as a sorbent and as an electrochemical sensor for heavy metal ions, thus opening a new window for materials with application in both environmental remediation and monitoring.
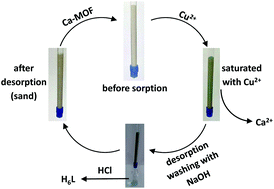
Cu2+ sorption from aqueous media by a recyclable Ca2+ framework.
Margariti A., Rapti S., Katsenis A.D., Friščić T., Georgiou Y., Manos M.J., Papaefstathiou G.S.Abstract
A new Ca2+ two-dimensional framework, namely [Ca(H4L)(DMA)2]·2DMA (Ca-MOF) was obtained from the initial use of N,N′-bis(2,4-dicarboxyphenyl)-oxalamide (H6L). We discovered that this Ca-MOF is capable of exchanging the Ca2+ ions by Cu2+ almost quantitatively in a matter of seconds in aqueous solution. The highly efficient Cu2+ sorption properties exhibited by the Ca-MOF were investigated in detail via batch ion-exchange studies. In addition, the Ca-MOF was utilized as a stationary phase in an ion-exchange column for Cu2+ removal from aqueous media. Furthermore, we were able to recycle the most expensive part of the framework, i.e. the H6L, by treating the column with dilute NaOH followed by HCl. The Ca-MOF represents the first example of any MOF capable of exchanging its constituent metal ions that is shown to be a highly effective ion-exchange material, thus opening a new window in the exploitation of MOFs for ion-exchange applications.
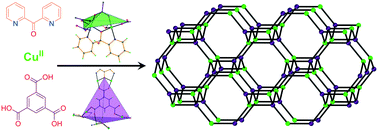
Cu(ii) frameworks from a “mixed-ligand” approach.
Fidelli A.M., Kessler V.G., Escuer A., Papaefstathiou G.S.Abstract
Employment of di-2-pyridyl ketone and poly-carboxylates in CuII chemistry afforded four complex hydrogen-bonded frameworks, one one-dimensional (1D), one 2D and three 3D coordination polymers. Di-2-pyridyl ketone underwent several metal-assisted transformations to yield three CuII structural units which, in combination with the poly-carboxylate anions of the trimesic, isophthalic, 5-hydroxy-isophthalic and pyromellitic acids, provided access to extended frameworks by dative or hydrogen bonds. All nine complex frameworks were realized in terms of their topological analysis. The 3D and the 2D polymers consisted of [Cu2] dimers and were found to be dominated by ferromagnetic interactions. The origin of the ferromagnetic coupling was attributed to the counter complementarity of the simultaneous alkoxo/syn,syn-carboxylate bridges within the dimers.
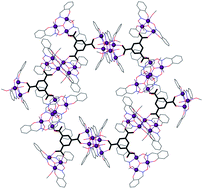
Two-dimensional frameworks built from Single-Molecule Magnets.
Katsenis A.D., Inglis R., Prescimone A., Brechin E.K., Papaefstathiou G.S.Abstract
Fine tuning the Mn/salicylaldoxime/trimesic acid reaction conditions leads to the formation of a regular 2D net held together by dative bonds and to a non-regular 2D net stabilised by both dative and hydrogen bonds. Both networks are built from [Mn6] Single-Molecule Magnets.
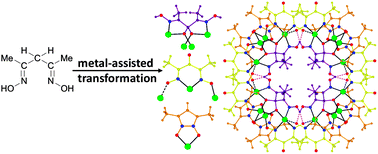
Circular serendipity: in situ ligand transformation for the self-assembly of an hexadecametallic [CuII16] wheel.
Kostopoulos A.K., Katsenis A.D., Frost J.M., Kessler V.G., Brechin E.K., Papaefstathiou G.S.Abstract
A [CuII16] wheel was isolated serendipitously from the reaction of acetylacetone dioxime with copper(II) chloride and lanthanide ions in a reaction initially designed to produce heterometallic 3d–4f cages. The ligand has been transformed in situ to three different forms, all found within the [Cu16] wheel, with the original ligand completely absent.
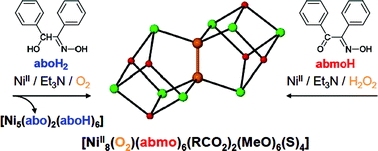
A family of [Ni8] cages templated by μ6-peroxide from dioxygen activation.
Perivolaris A., Stoumpos C.C., Karpinska J., Ryder A.G., Frost J.M., Mason K., Prescimone A., Slawin A.M.Z., Kessler V.G., Mathieson J.S., Cronin L., Brechin E.K., Papaefstathiou G.S.Abstract
A family of exceptionally thermally stable [Ni8] cages is reported, each being templated by a rare η3:η3:μ6-O22− species produced by dioxygen activation, where the reducing agent for the O2 reduction appears to be the ligand used in the reaction mixtures, which was found within the nickel cages in its oxidized form.
High-spin Ni(ii) clusters: triangles and planar tetranuclear complexes
Katsenis, A.D., Kessler, V.G., Papaefstathiou, G.S.Abstract
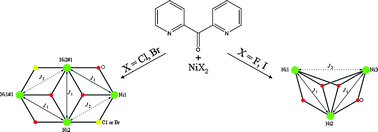
The exploration of the NiX2/py2CO/Et3N (X = F, Cl, Br, I; py2CO =