- Katsenis, A. D.; Brechin, E. K.; Papaefstathiou G. S.“Metal-Organic Frameworks From Single-Molecule Magnets” in Metal-Organic Framework Materials, Encyclopedia of Inorganic and Bioinorganic Chemistry, L. R. Macgillivray, C. Lukehart (Eds), John Wiley & Sons, 2014, 245-258.
- MacGillivray L. R.; Papaefstathiou G. S. “Solid-State Reactivity / Topochemistry” in Encyclopedia of Supramolecular Chemistry, J. L. Atwood, J. W. Steed (Eds), Marcel Dekker Inc. 2004, 1316-1321.
Book Chapters
- MacGillivray, L. R.; Papaefstathiou, G. S. "Gas Storage Materials and Devices", United States Patent 7481866
- MacGillivray, L. R.; Papaefstathiou, G. S. "Optical Data Storage Materials and Devices", 2004, provisional patent application (USA)
Patents
- Siaitanidou, C., Spanou, V., Chatzarakis, N. G., Oikonomopoulos, P., Papaefstathiou, G. S., Tsagaraki, K., Pelekanos, N. T. "Dual-Wavelength Lasing Due to Second Phase Inclusions in MAPbCl3." The Journal of Physical Chemistry C, 2025.
Article - Plessas, A. K., Fidelli, A. M., Kessler, V. G., Escuer, A., Papaefstathiou, G. S. "Synthesis, structure and magnetism of a low-symmetry [Ni4O4] cubane derived from di-2-pyridyl ketone and isophthalic acid and magnetostructural correlation." Journal of Coordination Chemistry, 2025, 1-14.
Article - Evangelou, D.A., Makri, E.C., Pliatsios, N., Vamvasakis, I., Buchsteiner, E., Oikonomopoulos, P., Armatas, G.S., Papaefstathiou, G. S., Lazarides, T. and Manos, M.J. "Ultramicroporous Al(iii) MOFs with selective CO2 adsorption, acid resistance, and efficient Cr(vi) sorption properties" Dalton Transactions, 2025, 54(36), 13658-13671.
Article - Anastasiadis, N.E., Karagianni, V.I., Kotidis, P., Dimitriou, C., Vamvasakis, I., Lazarides, T., Papaefstathiou, G. S., Deligiannakis, Y., Armatas, G.S., Buchsteiner, E. Manos, M.J. "Aqueously synthesized and reusable Ce (iv)-based metal–organic frameworks with aliphatic multicarboxylate linkers for highly efficient Pb2+ removal from water." Inorganic Chemistry Frontiers, 2025, 12(23), 7894-7906.
Article - Koukouviti, E., Plessas, A. K., Pagkali, V., Economou, A., Papaefstathiou, G. S., & Kokkinos, C. "3D-printed electrochemical glucose device with integrated Fe(II)-MOF nanozyme." Microchimica Acta, 2023, 190(7), 1-9.
Article - Oikonomopoulos, P., Pagkali, V., Kritikou, E., Panara, A., Kostakis, M. G., Thomaidis, N.S., Tziotzi, T. G., Economou, A., Kokkinos, C. & Papaefstathiou, G. S. "Oxalamide Based Fe(II)-MOFs as Potential Electrode Modifiers for Glucose Detection" Chemistry, 2023, 5(1), 19-30.
Article - Koukouviti, E., Plessas, A. K., Economou, A., Thomaidis, N., Papaefstathiou, G. S. & Kokkinos, C. "3D Printed Voltammetric Sensor Modified with an Fe(III)-Cluster for the Enzyme-Free Determination of Glucose in Sweat" Biosensors, 2022, 12(12), 1156.
Article - Vasiliou, F., Plessas, A. K., Economou, A., Thomaidis, N., Papaefstathiou, G. S. & Kokkinos, C. "Graphite paste sensor modified with a Cu(II)-complex for the enzyme-free simultaneous voltammetric determination of glucose and uric acid in sweat." J. Electroanal. Chem. 2022, 917, 116393.
Article - Moutzouris, N., Moushi, E. E., Tziotzi, T. G., Tarlas, G. D., Tasiopoulos, A. J., Escuer, A. & Papaefstathiou, G. S. "Metallo-ligand based 3d/4f coordination polymers: Synthesis, structure and magnetic properties." European Journal of Inorganic Chemistry, 2022.
Article - Fidelli, A. M., Katsenis, A. D., Kotidis, P., Tarlas, G. D., Pournara, A. & Papaefstathiou, G. S. "Enhanced Cr(VI) sorption capacity of the mechanochemically synthesized defective UiO-66 and UiO-66-NH2." J. Coord. Chem. 2021, 74, 2835–2849.
Article - Mertzenich, C. L., Papaefstathiou, G. S., Friščić, T., Hamilton, T. D., Bučar, D. K., Swenson, D. C., & MacGillivray, L. R. "Inverted metal–organic frameworks: isoreticular decoration with organic anions using principles of supramolecular chemistry". Journal of Coordination Chemistry, 2021, 74(1-3), 169-177.
Article - Margariti, A., Moushi, E., Tasiopoulos, A. J., Escuer, A. & Papaefstathiou, G. S. “Oxalamide based coordination polymers”. J. Coord. Chem., 2021, 74(1-3), 252-265.
Article - Diamantis, S.A., Hatzidimitriou, A., Plessas, A.K., Pournara, A., Manos, M.J., Papaefstathiou, G.S., Lazarides T. "Alkaline earth-organic frameworks with amino derivatives of 2,6-naphthalene dicarboxylates: structural studies and fluorescence properties". Dalton Transactions, 2020, 49, 16736-16744.
Article - Vlachou, E., Margariti, A., Papaefstathiou, G.S., Kokkinos, C. "Voltammetric determination of Pb(II) by a Ca-MOF-modified carbon paste electrode integrated in a 3D-printed device". Sensors (Switzerland), 2020, 20 (16), art. no. 4442, 1-10.
Communication - Kokkinos, C., Economou, A., Pournara, A., Manos, M., Spanopoulos, I., Kanatzidis, M., Tziotzi, T., Petkov, V., Margariti, A., Oikonomopoulos, P. & Papaefstathiou, G. S. "3D-printed lab-in-a-syringe voltammetric cell based on a working electrode modified with a highly efficient Ca-MOF sorbent for the determination of Hg(II)". Sensors Actuators B Chem., 2020, 321, 128508
Article - Moushi E.E., Kourtellaris A., Andreou E., Fidelli A., Papaefstathiou G.S., Plakatouras J.C., Tasiopoulos A.J. "New metal-organic frameworks derived from pyridine-3,5-dicarboxylic acid: Structural diversity arising from the addition of templates into the reaction systems" CrystEngComm, 2020 , 22, 11, 2083 - 2096
Article - Papazoi E., Douvali A., Diamantis S.A., Papaefstathiou G.S., Eliseeva S.V., Petoud S., Hatzidimitriou A.G., Lazarides T., Manos M.J. "Unravelling the mechanism of water sensing by the Mg2+ dihydroxy-terephthalate MOF (AEMOF-1′)" Molecular Systems Design and Engineering, 2020, 5, 2, 461 - 468
Article - Kourtellaris A., Moushi E.E., Spanopoulos I., Trikalitis P.N., Pissas M., Papaefstathiou G.S., Sanakis Y., Tasiopoulos A.J. "A Microporous Co(II)-Based 3-D Metal Organic Framework Built from Magnetic Infinite Rod-Shaped Secondary Building Units" European Journal of Inorganic Chemistry, 2019, 38, 4056 - 4062
Article - Pournara A. D., Tarlas G. D., Papaefstathiou G. S., & Manos M. J. "Chemically modified electrodes with MOFs for the determination of inorganic and organic analytes via voltammetric techniques: a critical review". Inorganic Chemistry Frontiers, 2019, 6(12), 3440-3455
Review - Pournara A.D., Margariti A., Tarlas G.D., Kourtelaris A., Petkov V., Kokkinos C., Economou A., Papaefstathiou G.S., Manos M.J. "A Ca2+ MOF combining highly efficient sorption and capability for voltammetric determination of heavy metal ions in aqueous media". Journal of Materials Chemistry A, 2019, 7, 25, 15432 - 15443
Article - Tarlas G.D., Katsenis A.D., Papaefstathiou G.S. "An I2O1 Barium Framework Derived from an In-Situ Metal-Assisted Ligand Transformation". Journal of Materials Chemistry A, 2018, 40, 4458 - 4464
Article - Margariti A., Pournara A.D., Manos M.J., Lazarides T., Papaefstathiou G.S. "Towards white-light emission by Tb3+/Eu3+ substitution in a Ca2+ framework". Polyhedron, 2018, 153, 24 - 30
Article - Diamantis S.A., Pournara A.D., Hatzidimitriou A.G., Manos M.J., Papaefstathiou G.S., Lazarides T. "Two new alkaline earth metal organic frameworks with the diamino derivative of biphenyl - 4,4' - dicarboxylate as bridging ligand: Structures, fluorescence and quenching by gas phase aldehydes". Polyhedron, 2018 , 153, 173 - 180
Article - Pournara A.D., Douvali A., Diamantis S., Papaefstathiou G.S., Hatzidimitriou A.G., Kaziannis S., Kosmidis C., Lazarides T., Manos M.J. "A new Cd2+ - dihydroxyterephthalate MOF: Synthesis, crystal structure and detailed photophysical studies" Polyhedron, 2018, 151, 401 - 406
Article - Diamantis S.A., Margariti A., Pournara A.D., Papaefstathiou G.S., Manos M.J., Lazarides T. "Luminescent metal-organic frameworks as chemical sensors: Common pitfalls and proposed best practices". Inorganic Chemistry Frontiers, 2018, 5, 7, 1493 - 1511
Review - Chu Q., Duncan A.J.E., Papaefstathiou G.S., Hamilton T.D., Atkinson M.B.J., Mariappan S.V.S., Macgillivray L.R. "Putting Cocrystal Stoichiometry to Work: A Reactive Hydrogen-Bonded "superassembly" Enables Nanoscale Enlargement of a Metal-Organic Rhomboid via a Solid-State Photocycloaddition". Journal of the American Chemical Society, 2018, 140, 14, 4940 - 4944
Article - Fidelli A.M., Armakola E., Demadis K.D., Kessler V.G., Escuer A., Papaefstathiou G.S. "Cu (II) Frameworks from Di- 2- pyridyl Ketone and Benzene-1, 3, 5- triphosphonic Acid". European Journal of Inorganic Chemistry, 2018, 1, 91 - 98
Article - Margariti A., Rapti S., Katsenis A.D., Friščić T., Georgiou Y., Manos M.J., Papaefstathiou G.S. "Cu2+ sorption from aqueous media by a recyclable Ca2+ framework". Inorganic Chemistry Frontiers, 2017, 4, 5, 773 - 781
Article - Birnara C., Kessler V.G., Papaefstathiou G.S. "Group (III) quinaldates: synthesis, structure and photoluminescence". Journal of Coordination Chemistry, 2017, 70, 6, 997 - 1007
Article - Papazoi E., Douvali A., Rapti S., Skliri E., Armatas G.S., Papaefstathiou G.S., Wang X., Huang Z.-F., Kaziannis S., Kosmidis C., Hatzidimitriou A.G., Lazarides T., Manos M.J. "A microporous Mg2+ MOF with cation exchange properties in a single- crystal- to- single- crystal fashion". Inorganic Chemistry Frontiers, 2017, 4, 3, 530 - 536
Article - Fidelli A.M., Kessler V.G., Escuer A., Papaefstathiou G.S. "Cu(II) frameworks from a "mixed-ligand" approach". CrystEngComm, 2017, 19, 30, 4355 - 4367
Article - Kourtellaris A., Moushi E.E., Spanopoulos I., Tampaxis C., Charalambopoulou G., Steriotis T.A., Papaefstathiou G.S., Trikalitis P.N., Tasiopoulos A.J. "A microporous Cu2+ MOF based on a pyridyl isophthalic acid Schiff base ligand with high CO2 uptake". Inorganic Chemistry Frontiers, 2016, 3, 12, 1527 - 1535
Article - Douvali A., Papaefstathiou G.S., Gullo M.P., Barbieri A., Tsipis A.C., Malliakas C.D., Kanatzidis M.G., Papadas I., Armatas G.S., Hatzidimitriou A.G., Lazarides T., Manos M.J. "Alkaline Earth Metal Ion/ Dihydroxy- Terephthalate MOFs: Structural Diversity and Unusual Luminescent Properties". Inorganic Chemistry, 2015, 54, 12, 5813 - 5826
Article - Douvali A., Tsipis A.C., Eliseeva S.V., Petoud S., Papaefstathiou G.S., Malliakas C.D., Papadas I., Armatas G.S., Margiolaki I., Kanatzidis M.G., Lazarides T., Manos M.J. "Turn-on luminescence sensing and real-time detection of traces of water in organic solvents by a flexible metal-organic framework." Angewandte Chemie - International Edition, 2015, 54, 5, 1651 - 1656
Article - Moushi E.E., Kourtellaris A., Spanopoulos I., Manos M.J., Papaefstathiou G.S., Trikalitis P.N., Tasiopoulos A.J. "A microporous Co2+ metal organic framework with single-crystal to single-crystal transformation properties and high CO2 uptake". Crystal Growth and Design, 2015, 15, 1, 185 - 193
Article - Papaefstathiou G.S., Duncan A.J.E., Mac Gillivray L.R. "Two act as one: unexpected dimers of catechol direct a solid-state [2+2] photodimerization in a six-component hydrogen-bonded assembly". Chemical Communications, 2014, 50, 100, 15960 - 15962
Article - Kostopoulos A.K., Katsenis A.D., Frost J.M., Kessler V.G., Brechin E.K., Papaefstathiou G.S. "Circular serendipity: In situ ligand transformation for the self-assembly of an hexadecametallic [CuII16] wheel". Chemical Communications, 2014, 50, 95, 15002 - 15005
Article - Perivolaris A., Stoumpos C.C., Karpinska J., Ryder A.G., Frost J.M., Mason K., Prescimone A., Slawin A.M.Z., Kessler V.G., Mathieson J.S., Cronin L., Brechin E.K., Papaefstathiou G.S. "A family of [Ni8] cages templated by μ6-peroxide from dioxygen activation". Inorganic Chemistry Frontiers, 2014, 1, 6, 487 - 494
Article - Papaefstathiou G.S., Subrahmanyam K.S., Armatas G.S., Malliakas C.D., Kanatzidis M.G., Manos M.J. "A unique microporous copper trimesate selenite with high selectivity for CO2". CrystEngComm, 2014, 16, 17, 3483 - 3486
Article - Perivolaris A., Fidelli A.M., Inglis R., Kessler V.G., Slawin A.M.Z., Brechin E.K., Papaefstathiou G.S. "A family of hexanuclear Mn(III) single-molecule magnets". Journal of Coordination Chemistry, 2014, 67, 23 - 24, 3972 - 3986
Article - Tella S., Bekiari V., Kessler V.G., Papaefstathiou G.S. "Gallium(III) complexes based on N, N′- bis(salicylidene)propane- 1, 3- diamine and its derivatives". Polyhedron, 2013, 64, 77 - 83
Article - Manoli M., Inglis R., Manos M.J., Papaefstathiou G.S., Brechin E.K., Tasiopoulos A.J. "A 1-D coordination polymer based on a Mn40 octagonal super-structure". Chemical Communications, 2013, 49, 11, 1061 - 1063
Article - Kyprianidou E.J., Papaefstathiou G.S., Manos M.J., Tasiopoulos A.J. "A flexible Cd2+ metal organic framework with a unique (3, 3, 6)-connected topology, unprecedented secondary building units and single crystal to single crystal solvent exchange properties". CrystEngComm, 2012, 14, 24, 8368 - 8373
Article - Manos M.J., Moushi E.E., Papaefstathiou G.S., Tasiopoulos A.J. "New Zn2+ metal organic frameworks with unique network topologies from the combination of trimesic acid and amino-alcohols". Crystal Growth and Design, 2012, 12, 11, 5471 - 5480
Article - Manos M.J., Kyprianidou E.J., Papaefstathiou G.S., Tasiopoulos A.J. "Insertion of functional groups into a Nd3+ metal-organic framework via single-crystal-to-single-crystal coordinating solvent exchange". Inorganic Chemistry, 2012, 51 , 11, 6308 - 6314
Article - Katsenis A.D., Inglis R., Prescimone A., Brechin E.K., Papaefstathiou G.S. "Two-dimensional frameworks built from Single-Molecule Magnets". CrystEngComm, 2012, 14, 4, 1216 - 1218
Article - Manos, M.J., Markoulides, M.S., Malliakas, C.D., Papaefstathiou, G.S., Chronakis, N., Kanatzidis, M.G., Trikalitis, P.N., Tasiopoulos, A.J. "A highly porous interpenetrated metal-organic framework from the use of a novel nanosized organic linker". Inorganic Chemistry, 2011, 50 (22), 11297-11299
Article - Katsenis, A.D., Kessler, V.G., Papaefstathiou, G.S. "High-spin Ni(ii) clusters: Triangles and planar tetranuclear complexes". Dalton Transactions, 2011, 40 (17), 4590-4598
Article - Mason, K., Gass, I.A., White, F.J., Papaefstathiou, G.S., Brechin, E.K., Tasker, P.A. "Hexa- and octanuclear iron(iii) salicylaldoxime clusters". Dalton Transactions, 2011, 40 (12), 2875-2881
Article - Inglis, R., White, F., Piligkos, S., Wernsdorfer, W., Brechin, E.K., Papaefstathiou, G.S. "Chiral single-molecule magnets: A partial Mn(III) supertetrahedron from achiral components". Chemical Communications, 2011, 47 (11), 3090-3092
Article - Papaefstathiou, G.S., Drakopoulou, L., Raptopoulou, C.P., Terzis, A. "Hydrogen-bonded networks based on cobalt(ii), nickel(ii), and zinc(II) Complexes of N,N'-Diethylurea". Bioinorganic Chemistry and Applications, 2010, art. no. 618202
Article - Kyriakidis, D.A., Zanias, S., Papaefstathiou, G.S., Raptopoulou, C.P., Papazisis, K.T., Vala, V., Zambouli, D., Kortsaris, A.H., Zafiropoulos, T.F. "Synthesis, structure, and antiproliferative activity of three gallium(III) azole complexes". Bioinorganic Chemistry and Applications, 2010, art. no. 168030
Article - Inglis, R., Katsenis, A.D., Collins, A., White, F., Milios, C.J., Papaefstathiou, G.S., Brechin, E.K. "Assembling molecular triangles into discrete and infinite architectures". CrystEngComm, 2010, 12 (7), 2064-2072
Article - Meally, S.T., McDonald, C., Karotsis, G., Papaefstathiou, G.S., Brechin, E.K., Dunne, P.W., McArdle, P., Power, N.P., Jones, L.F. "A family of double-bowl pseudo metallocalix[6]arene discs". Dalton Transactions, 2010 ,39 (20), 4809-4816
Article - Sartzi, H., Papaefstathiou, G.S., Psycharis, V., Escuer, A., Perlepes, S.P., Stoumpos, C.C. "Halo and azido copper(II) coordination polymers featuring the gem-diolate forms of di-2-pyridyl ketone". Polyhedron, 2010, 29 (1), 100-109
Article - Meally, S.T., Karotsis, G., Brechin, E.K., Papaefstathiou, G.S., Dunne, P.W., McArdle, P., Jones, L.F. "Planar [Ni7] discs as double-bowl, pseudo metallacalix[6]arene host cavities". CrystEngComm, 2010, 12 (1), 59-63
Article - Inglis, R., Taylor, S.M., Jones, L.F., Papaefstathiou, G.S., Perlepes, S.P., Datta, S., Hill, S., Wernsdorfer, W., Brechin, E.K. "Twisting, bending, stretching: Strategies for making ferromagnetic [Mn3III] triangles". Dalton Transactions, 2009, (42), 9157-9168
Article - Birnara, C., Kessler, V.G., Papaefstathiou, G.S. "Mononuclear gallium(III) complexes based on salicylaldoximes: Synthesis, structure and spectroscopic characterization". Polyhedron, 2009, 28 (15), 3291-3297
Article - Milios, C.J., Ioannou, P.V., Raptopoulou, C.P., Papaefstathiou, G.S. "Crystal engineering with 2,1,3-benzoselenadiazole and mercury(II) chloride". Polyhedron, 2009, 28 (15), 3199-3202
Article - Kagkelari, A., Papaefstathiou, G.S., Raptopoulou, C.P., Zafiropoulos, T.F. "Synthesis and structure of N-salicylidene-o-aminophenolato gallium(III) complexes". Polyhedron, 2009, 28 (15), 3279-3283
Article - Katsoulakou, E., Papaefstathiou, G.S., Konidaris, K.F., Pairas, G., Raptopoulou, C., Cordopatis, P., Manessi-Zoupa, E. "Synthesis, structural study and topological analysis of Zn/Aib and Aib-based small peptide complexes (H-Aib-OH = α-aminoisobutyric acid)". Polyhedron, 2009, 28 (15), 3387-3399
Article - Inglis, R., Papaefstathiou, G.S., Wernsdorfer, W., Brechin, E.K. "Ferromagnetic [Mn3] single-molecule magnets and their supramolecular networks". Australian Journal of Chemistry, 2009, 62 (9), 1108-1118
Conference Paper - Katsenis, A.D., Inglis, R., Slawin, A.M.Z., Kessler, V.G., Brechin, E.K., Papaefstathiou, G.S. "Transforming the cube: A tetranuclear cobalt(II) cubane cluster and its transformation to a dimer of dimers". CrystEngComm, 2009, 11 (10), 2117-2120
Article - Konidaris, K.F., Papaefstathiou, G.S., Aromi, G., Teat, S.J., Manessi-Zoupa, E., Escuer, A., Perlepes, S.P. "A three-dimensional copper(II) coordination polymer featuring the 2,3-dioxyquinoxalinate(-2) ligand: Preparation, structural characterization and magnetic study". Polyhedron, 2009, 28 (9-10), 1646-1651
Article - Stamatatos, T.C., Vlahopoulou, J.C., Tangoulis, V., Raptopoulou, C.P., Terzis, A., Papaefstathiou, G.S., Perlepes, S.P. "New copper(II) clusters and coordination polymers from the amalgamation of azide/benzoate/di-2-pyridyl ketone ligands". Polyhedron, 2009, 28 (9-10), 1656-1663
Article - Karotsis, G., Stoumpos, C., Collins, A., White, F., Parsons, S., Slawin, A.M.Z., Papaefstathiou, G.S., Brechin, E.K. "Molecular and supramolecular Ni(II) wheels from α-benzoin oxime". Dalton Transactions, 2009, (18), 3388-3390
Article - Kagkelari, A., Bekiari, V., Stathatos, E., Papaefstathiou, G.S., Raptopoulou, C.P., Zafiropoulos, T.F., Lianos, P. "Photoluminescence and electroluminescence by gallium(III) complexes of N-salicylidene-o-aminophenol and its derivatives". Journal of Luminescence, 2009, 129 (5), 578-583
Article - Katsenis, A.D., Lalioti, N., Bekiari, V., Lianos, P., Raptopoulou, C.P., Terzis, A., Perlepes, S.P., Papaefstathiou, G.S. "Initial use of 1-hydroxybenzotriazole in the chemistry of group 12 metals: An 1D zinc(II) coordination polymer and a mononuclear cadmium(II) complex containing the deprotonated ligand in a novel monodentate ligation mode". Inorganic Chemistry Communications, 2009, 12 (2), 92-96
Article - Stoumpos, C.C., Inglis, R., Karotsis, G., Jones, L.F., Collins, A., Parsons, S., Milios, C.J., Papaefstathiou, G.S., Brechin, E.K. "Supramolecular entanglement from interlocked molecular nanomagnets". Crystal Growth and Design, 2009, 9 (1), 24-27
Article - Hamilton, T.D., Papaefstathiou, G.S., Friščić, T., Bučar, D.-K., MacGillivray, L.R. "Onion-shell metal-organic polyhedra (MOPs): A general approach to decorate the exteriors of MOPs using principles of supramolecular chemistry". Journal of the American Chemical Society, 2008, 130 (44), 14366-14367
Article - Stamatatos, T.C., Tangoulis, V., Raptopoulou, C.P., Terzis, A., Papaefstathiou, G.S., Perlepes, S.P. "Di-2-pyridyl ketone/benzoate/azide combination as a source of copper(II) clusters and coordination polymers: Dependence of the product identity on the solvent". Inorganic Chemistry, 2008, 47 (18), 7969-7971
Article - Karotsis, G., Jones, L.F., Papaefstathiou, G.S., Collins, A., Parsons, S., Nguyen, T.D., Evangelisti, M., Brechin, E.K. "Rare tetranuclear mixed-valent [MnII2MnIV2] clusters as building blocks for extended networks". Dalton Transactions, 2008, (36), 4917-4925
Article - Katsoulakou, E., Tiliakos, M., Papaefstathiou, G.S., Terzis, A., Raptopoulou, C., Geromichalos, G., Papazisis, K., Papi, R., Pantazaki, A., Kyriakidis, D., Cordopatis, P., Manessi-Zoupa, E. "Diorganotin(IV) complexes of dipeptides containing the α-aminoisobutyryl residue (Aib): Preparation, structural characterization, antibacterial and antiproliferative activities of [(n-Bu)2Sn(H-1L)] (LH = H-Aib-L-Leu-OH, H-Aib-L-Ala-OH)". Journal of Inorganic Biochemistry, 2008, 102 (7), 1397-1405
Article - Macgillivray, L.R., Papaefstathiou, G.S., Friščić, T., Hamilton, T.D., Bučar, D.-K., Chu, Q., Varshney, D.B., Georgiev, I.G. "Supramolecular control of reactivity in the solid state: From templates to ladderanes to metal-organic frameworks". Accounts of Chemical Research, 2008, 41 (2), 280-291
Article - Bučar, D.-K., Papaefstathiou, G.S., Hamilton, T.D., MacGillivray, L.R. "A lanthanide-based helicate coordination polymer derived from a rigid monodentate organic bridge synthesized in the solid state". New Journal of Chemistry, 2008, 32 (5), 797-799
Article - Drakopoulou, L., Papatriantafyllopoulou, C., Terzis, A., Perlepes, S.P., Manessi-Zoupa, E., Papaefstathiou, G.S. "Synthesis, X-ray structure, and characterization of a complex containing the hexakis(urea)cobalt(II) cation and lattice urea molecules". Bioinorganic Chemistry and Applications, 2007, art. no. 51567
Article - Bučar, D.-K., Papaefstathiou, G.S., Hamilton, T.D., Chu, Q.L., Georgiev, I.G., MacGillivray, L.R. "Template-controlled reactivity in the organic solid state by principles of coordination-driven self-assembly". European Journal of Inorganic Chemistry, 2007, (29), 4559-4568
Short Survey - Stamatatos, T.C., Papaefstathiou, G.S., MacGillivray, L.R., Escuer, A., Vicente, R., Ruiz, E., Perlepes, S.P. "Ferromagnetic coupling in a 1D coordination polymer containing a symmetric [Cu(μ1,1-N3)2Cu(μ1,1-N3)2Cu]2+ core and based on an organic ligand obtained from the solid state". Inorganic Chemistry, 2007, 46 (21), 8843-8850
Article - Milios, C.J., Raptopoulou, C.P., Terzis, A., Perlepes, S.P., Papaefstathiou, G.S. "A mononuclear and a mixed-valence chain polymer arising from copper(II) halide chemistry and the use of 2,2′-pyridil". Bioinorganic Chemistry and Applications, 2007, art. no. 28508
Article - Papaefstathiou, G.S., Sofetis, A., Raptopoulou, C.P., Terzis, A., Spyroulias, G.A., Zafiropoulos, T.F. "2,2′-Bipyridine,1,10-phenanthroline and 2,2′:6′,2″-terpyridine in gallium(III) chemistry: Complexes containing the {GaIII2 (μ -OH)2}4+ core". Journal of Molecular Structure, 2007, 837 (1-3), 5-14
Article - Papaefstathiou, G.S., Boudalis, A.K., Stamatatos, T.C., Milios, C.J., Efthymiou, C.G., Raptopoulou, C.P., Terzis, A., Psycharis, V., Sanakis, Y., Vicente, R., Escuer, A., Tuchagues, J.-P., Perlepes, S.P. "A general synthetic route for the preparation of high-spin molecules: Replacement of bridging hydroxo ligands in molecular clusters by end-on azido ligands". Polyhedron, 2007, 26 (9-11), 2089-2094
Article - Papaefstathiou, G.S., Manessi, A., Raptopoulou, C.P., Terzis, A., Zafiropoulos, T.F. "Methanolysis as a route to gallium(III) clusters: Synthesis and structural characterization of a decanuclear molecular wheel". Inorganic Chemistry, 2006, 45 (22), 8823-8825
Article - Hamilton, T.D., Bučar, D.-K., Atkinson, M.B.J., Papaefstathiou, G.S., MacGillivray, L.R. "1D and 2D metal-organic frameworks functionalized with free pyridyl groups". Journal of Molecular Structure, 2006, 796 (1-3), 58-62
Article - Hamilton T. D.; Papaefstathiou G.S.; MacGillivray L. R. "Bis-Chelation and Anion Effects Involving a Molecule Constructed in the Organic Solid State Using Molecular Templates". American Crystallographic Association, Etter Transactions 2005, 1, 2-5
Article - Friščić, T., Hamilton, T.D., Papaefstathiou, G.S., MacGillivray, L.R. "A template-controlled solid-state reaction for the organic chemistry laboratory". Journal of Chemical Education, 2005, 82 (11), 1679-1681Article
- Papaefstathiou, G.S., Friščić, T., MacGillivray, L.R. "Design and construction of a 2D metal organic framework with multiple cavities: A nonregular net with a paracyclophane that codes for multiply fused nodes". Journal of the American Chemical Society, 2005, 127 (41), 14160-14161
Article - Papaefstathiou, G.S., Georgiev, I.G., Friščić, T., MacGillivray, L.R. "Directed assembly and reactivity of olefins within a one-dimensional ladder-like coordination polymer based on a dinuclear Zn(II) platform". Chemical Communications, 2005, (31), 3974-3976
Article - Papaefstathiou, G.S., Escuer, A., Mautner, F.A., Raptopoulou, C., Terzis, A., Perlepes, S.P., Vicente, R. "Use of the di-2-pyridyl ketone/acetate/dicyanamide "blend" in manganese(II), cobalt(II) and nickel(II) chemistry: Neutral cubane complexes". European Journal of Inorganic Chemistry, 2005, (5), 879-893
Article - Hamilton, T.D., Papaefstathiou, G.S., MacGillivray, L.R. "Template-controlled reactivity: Following nature's way to design and construct metal-organic polyhedra and polygons". Journal of Solid State Chemistry, 2005, 178 (8 SPEC. ISS.), 2409-2413
Article - Papaefstathiou G. S., Friscic T., MacGillivray L. R. “An Infinite Hydrogen-Bonded Molecular Assembly based on Catechol and a Bifunctional Olefin”. Transactions of the American Crystallographic Association 2004, 39, 110-113
Communication - Manessi A., Papaefstathiou G. S., Raptopoulou C. P., Terzis A., Zafiropoulos Th. F. “Synthetic Analogue Approach to Metallobleomycins: Syntheses, Structure and Properties of Mononuclear and Tetranuclear Gallium(III) Complexes of a Ligand that Resembles the Metal Binding Site of Bleomycin”. Journal of Inorganic Biochemistry 2004, 98, 2052-2062
Article - MacGillivray L. R., Papaefstathiou G. S., Friscic T., Varshney D. B., Hamilton T. D. "Template-Controlled Synthesis in the Solid-State". Topics in Current Chemistry 2004, 248, 201-221
Review - Papaefstathiou G. S., Zhong Z., Geng L., MacGillivray L. R. "Coordination-Driven Self-Assembly Directs a Single-Crystal-to-Single-Crystal Transformation that Exhibits Photocontrolled Fluorescence" Journal of the American Chemical Society 2004, 126, 9158-9159
Communication - Sofetis A., Papaefstathiou G. S., Terzis A., Raptopoulou C. P., Zafiropoulos T. F. "Preparation, Crystal Structure and Spectroscopic Characterization of [Ga(OH)(SO4)(terpy)(H2O)]·H2O (terpy = 2,2΄:6΄,2-Terpyridine): The First Characterized Gallium(III) Sulfato Complex". Zeitschrift für Naturforschung b 2004, 59, 291-297
Article - Gao X., Friscic T. Papaefstathiou G. S., MacGillivray L. R. "Crystal and Molecular Structure of Rebek’s Imide". Journal of Chemical Crystallography 2004, 34, 171-174
Article - Papaefstathiou G. S., Milios C. J., MacGillivray L. R. "A 2D Metal-Organic Framework with Two Different Rhombus-Shaped Cavities: A Rare Example of a (4,4)-Net with Alternating Metal and Organic Nodes". Microporous and Mesoporous Materials 2004, 71, 11-15
Article - Papaefstathiou G. S., Hamilton T. D., Friscic T., MacGillivray L. R. "Self-Assembled Metal-Organic Squares Derived from Linear Templates as Exemplified by a Polydentate Ligand that Provides Access to Both a Polygon and Polyhedron". Chemical Communications 2004, 270-271
Communication - Papaefstathiou G. S., Manessi S., Raptopoulou C. P., Behrman E. J., Zafiropoulos Th. F. "The First Metal Complex of 5-hydroxyorotic Acid : Dimethylammonium bis (N,N-dimethylformamide) bis(5-hydroxyorotato(-2))gallate(III)". Inorganic Chemistry Communications 2004, 7, 69-72
Communication - Papaefstathiou G. S., MacGillivray L. R. “Inverted Metal-Organic Frameworks: Molecular Solid-State Materials with Modular Functionality”. Coordination Chemistry Reviews 2003, 246, 169-184
Special Issue “Structure, Properties and Applications of Inorganic Polymers”
Review - Keuleers R., Desseyn H. O., Papaefstathiou G. S., Drakopoulou L., Perlepes S. P., Raptopoulou C. P., Terzis A. “Hydrogen Bonded Networks Based on Manganese(II), Nickel(II), Copper(II) and Zinc(II) Complexes of N,N’-dimethylurea”. Transition Metal Chemistry 2003, 28, 548-557
Article - Diamantopoulou E., Papaefstathiou G. S., Terzis A., Raptopoulou C. P., Desseyn H. O., Perlepes S. P. “Hydrogen Bonded Networks Based on Lanthanide(III) Complexes of N,N’-dimenthylurea (DMU) : Preparation, Characterisation and Crystal Structures of [Nd(DMU)6] [NdCl6] and [Nd(NO3)3(DMU)3]”. Polyhedron 2003, 22, 825-835
Article - Papaefstathiou G. S., Keuleers R., Milios C. J., Raptopoulou C. P., Terzis A., Desseyn H. O., Perlepes S. P. “Hexakis(N,N’-dimethylurea)cobalt(II) Cation : A Flexible Building Block for the Construction of Hydrogen Bonded Networks”. Zeitschrift für Naturforschung b 2003, 58, 74-84
Article - Papaefstathiou G. S., Friscic T., MacGillivray L. R. "A Regiocontrolled ‘Head-to-Tail’ [2+2] Photodimerization of a Stilbene Involving a Ternary Solid Based on Catechol". Journal of Supramolecular Chemistry 2002, 2, 227-231
Article - De Beukeleer S. , Papaefstathiou G. S. , Raptopoulou C. P. , Terzis A. , Perlepes S. P. , Desseyn H. O. , Manessi-Zoupa E. "Studies of Monothiomalonamide Nitrate Palladium(II) and Platinum(II) Complexes". Zeitschrift für Naturforschung b 2002, 57, 1224-1236
Article - Papaefstathiou G. S. , Escuer A. , Font-Bardía M. , Perlepes S. P. , Solans X. , Vicente R. "Benzoate as Terminal Ligand in the Defective Double-Cubane, Tetranuclear Cobalt(II) Complex [Co4(N3)2(O2CPh)2{(py)2C(OH)O}4]·2DMF with Simultaneous μ1,1-azido and μ-O Bridges [(py)2C(OH)O = the Monoanion of the Hydrated, gem-diol Form of Di-2-pyridyl Ketone". Polyhedron 2002, 21, 2027-2032
Article - Hamilton T. D., Papaefstathiou G. S., MacGillivray L. R. "A Polyhedral Host Constructed Using a Linear Template". Journal of the American Chemical Society 2002, 124, 11606-11607
Communication - Papaefstathiou G. S., Darrow B. G., MacGillivray L. R. "Crystal and Molecular Structure of [Cu2(3,5-dihydroxybenzoate)4(acetonitrile)2]·8H2O". Journal of Chemical Crystallography 2002, 32, 191-195
Article - Hamilton T. D., Papaefstathiou G. S., MacGillivray L. R. "Discrete and Infinite Coordination Arrays Derived from a Template-directed Solid-state Organic Synthesis". CrystEngComm. 2002, 4, 223-226
Communication - Varshney D. B., Papaefstathiou G. S., MacGillivray L. R. "Site-Directed Regiocontrolled Synthesis of a "Head-to-Head" Photodimer via a Single Crystal to Single-Crystal Transformation Involving a Linear Template". Chemical Communications 2002, 1964-1965
Communication - Papaefstathiou G. S., Vicente R., Raptopoulou C. P., Terzis A., Escuer A., Perlepes S. P. "A One Dimensional Manganese(II) Coordination Polymer Derived from Zerovalent Manganese and 1-Hydroxybenzotriazole – Synthesis, Characterization, Crystal Structure and Magnetic Properties". European Journal of Inorganic Chemistry 2002, 2488-2493
Article - Papaefstathiou G. S., Perlepes S. P. "Families of Polynuclear Manganese, Cobalt, Nickel and Copper Complexes Stabilized by Various Forms of Di-2-pyridyl Ketone". Comments on Inorganic Chemistry 2002, 23, 249-274
Review - Papaefstathiou G. S., MacGillivray L. R. "An Inverted Metal-Organic Framework with Compartmentalized Cavities Constructed by Using an Organic Bridging Unit Derived from the Solid State". Angewandte Chemie International Edition 2002, 41, 2070-2073
Communication - MacGillivray L. R., Reid J. L., Ripmeester J. A., Papaefstathiou G. S. "Toward a Reactant Library in Template-Directed Solid-State Organic Synthesis: Reactivity Involving Mono-Functionalized Reactant Based on a Stilbazole". Industrial and Engineering Chemistry Research 2002, 41, 4494-4497
Article - Skorda K., Papaefstathiou G. S., Vafiadis A., Lithoxoidou A., Raptopoulou C. P., Terzis A., Psycharis V., Bakalbassis E., Tangoulis V., Perlepes S. P. "The [Cu2(O2CMe)4(btd)2] Complex as a Bridging Unit : Preparation, Characterization, X-ray Structure and Magnetism of the 2D Coordination Polymer {[Cu6(O2CMe)8(OMe)4(btd)2]}n (btd = 2,1,3-benzothiadiazole)". Inorganica Chimica Acta 2001, 326, 53-64
Special Issue In memory of Prof. Olivier Kahn
Article - Papaefstathiou G. S., MacGillivray L. R. "Discrete Versus Infinite Molecular Self-Assembly: Control in Crystalline Hydrogen-Bonded Assemblies Based on Resorcinol". Organic Letters 2001, 3, 3835-3838
Communication - Papaefstathiou G. S., Kipp A. J., MacGillivray L. R. "Exploiting Modularity in Template-Controlled Synthesis: a New Linear Template to Direct Reactivity within Discrete Hydrogen-Bonded Molecular Assemblies in the Solid-State". Chemical Communications 2001, 2462-2463
Communication - Papaefstathiou G. S., Escuer A., Vicente R., Font-Bardia M., Solans X., Perlepes S. P. "Reactivity in Polynuclear Transition Metal Chemistry as a Means to Obtain High-Spin Molecules: Substitution of μ4-OH- by η1, μ4-N3- Increases Nine Times the Ground-State S Value of a Nonanuclear Nickel(II) Cage". Chemical Communications 2001, 2414-2415
Communication - MacGillivray L. R., Papaefstathiou G. S., Reid J. L., Ripmeester J. A. "A Rod-Shaped Guest Leads to Architectural Isomerism in a Multicomponent Crystalline Framework Based on a Resorcin[4]arene". Crystal Growth & Design 2001, 1, 373-375
Article - Papaefstathiou G. S., Perlepes S. P., Escuer A., Vicente R., Gantis A., Raptopoulou C. P., Tsohos A., Psycharis V., Terzis A., Bakalbassis E. G. "Topological Control in Two-Dimensional Cobalt(II) Coordination Polymers by p-p Stacking Interactions: Synthesis, Spectroscopic Characterization, Crystal Structure and Magnetic Properties". Journal of Solid State Chemistry 2001, 159, 371-378
Special Issue "New Horizons for Magnetic Solids Based on Molecules: From High-Tc Magnets to Nanomagnets to Devices", a tribute to Prof. Olivier Kahn
Article - Papaefstathiou G. S., Tsohos A., Raptopoulou C. P., Terzis A., Psycharis V., Gatteschi D., Perlepes S. P. "Crystal Engineering: Stacking Interactions Control the Crystal Structures of Benzothiadiazole (btd) and its Complexes With Copper(II) and Copper(I) Chlorides". Crystal Growth & Design 2001, 1,191-194
Communication - Papaefstathiou G. S., Escuer A., Raptopoulou C. P., Terzis A., Perlepes S. P., Vicente R. "Defective Double-Cubane Tetranuclear Manganese(II) and Cobalt(II) Complexes with Simultaneous μ1,1-Azido and m-O Bridges". European Journal of Inorganic Chemistry 2001, 1567-1574
Article - Papaefstathiou G. S., Perlepes S. P., Escuer A., Vicente R., Font-Bardia M., Solans X. "Unique Single-Atom Binding of Pseudohalogeno Ligands to Four Metal Ions Induced by their Trapping into High Nuclearity Cages". Angewandte Chemie International Edition 2001, 40, 884-886
Communication - Papaefstathiou G. S., Peeters A., Lenstra A. T. H., Desseyn H. O., Perlepes S. P. "Structural and Spectral Studies of N-alkyloxamates and their Complexes: X-ray Structures of MeHNCOCOOK and [Cu(EtHNCOCOO)2], and Vibrational Studies". Journal of Molecular Structure 2001, 559, 167-177
Article - Papaefstathiou G. S., Raptopoulou C. P., Tsohos A., Terzis A., Bakalbassis E. G., Perlepes S. P. "Alcoholysis of 2,2´-pyridil, (2-C5H4N)C(O)C(O)(2-C5H4N), in the Presence of Copper(II): A Family of Planar Pentanuclear Copper(II) Complexes Stabilized by [(2-C5H4N)C(O)(OR)C(O)(OR)(2-C5H4N)]2- and Carboxylate Ligands". Inorganic Chemistry 2000, 39, 4658-4662
Note - Keuleers R., Papaefstathiou G. S., Raptopoulou C. P., Desseyn H. O., Perlepes S. P. "Comparative Study of the Metal-ligand Bond Strength in Mn(II)/X/U Complexes (X = Cl, Br, I, U = urea)". Journal of Molecular Structure 2000, 525, 173-183
Article - Keuleers R., Papaefstathiou G. S., Raptopoulou C. P., Tangoulis V., Desseyn H. O., Perlepes S. P. "Tris(N,N´-dimethylurea)bis(Nitrate-O,O´)manganese(II), the First Example of a Seven-coordinate Manganese(II) Complex with a Monodentate Organic Ligand". Inorganic Chemistry Communications 1999, 2, 472-475
Communication
Publications
2025
Abstract
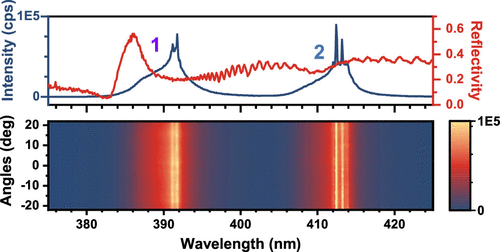
We report for the first time dual-wavelength lasing at 78 K in a vertical cavity containing ultrasmooth MAPbCl3 single crystals. To understand this unusual lasing behavior, the MAPbCl3 single crystals were thoroughly investigated in terms of temperature-dependent optical experiments. Microreflectivity measurements reveal, besides the standard exciton feature at the ∼385 nm orthorhombic bandgap, an additional previously unreported excitonic peak at ∼412 nm. This second exciton feature aligns with the second lasing line, strongly suggesting the coexistence of a “second phase” within the primary orthorhombic lattice. We show that this second phase persists up to 300 K and is likely associated with the quantum dot-like nanostructures detected on the surface of the MAPbCl3 crystals. The second phase depends on the growth method and crystal size but is likely traceable in all MAPbCl3 systems. These insights offer enhanced understanding of the MAPbCl3 system and open new pathways for blue-UV photonic devices.
Abstract
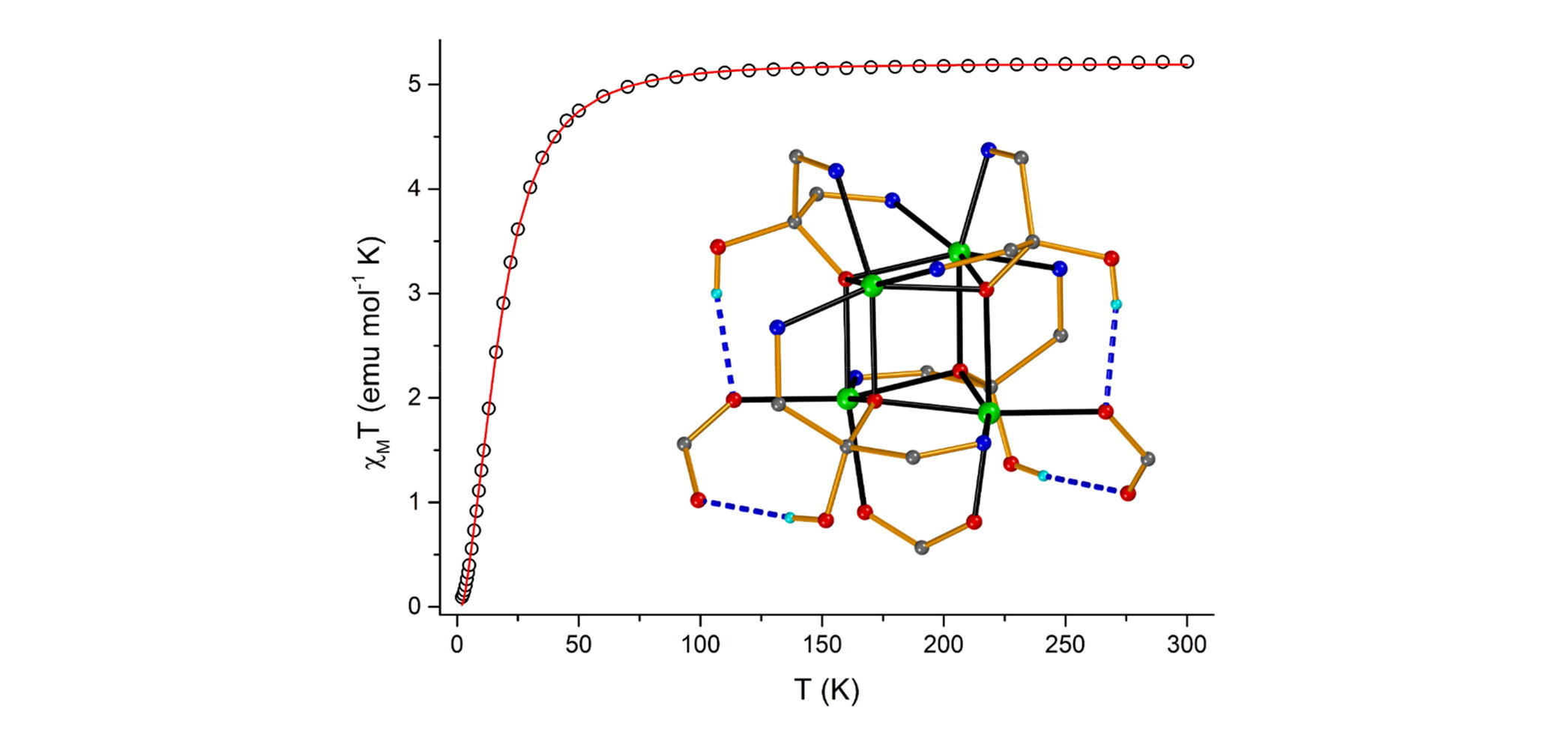
In an attempt to expand our “mixed-ligand” synthetic approach for the isolation of new coordination polymers from the simultaneous use of di-2-pyridyl ketone (py2CO) and isophthalic acid (H2bdc), we isolated a new tetranuclear complex with a low-symmetry [Ni4O4] cubane core and pending RCOOH/RCOO– groups, namely [Ni4{py2CO(OH)}4(bdc)(Hbdc)2]·0.5H2O (1), (py2C(OH)2 is the hydrate of py2CO). In this distorted [Ni4(μ3-OR)4]4+, which comprises the deprotonated O atoms of the py2C(OH)O– ligands, one of the six Ni···Ni distances is relatively short (2.965 Å) with the rest ranging from 3.029 − 3.292 Å. The six [Ni2O2] faces of the cube are divided in three pairs of opposite faces, with large (∼103°) and small (∼93.2°) Ni-O-Ni angles, and one pair with a small and a large Ni-O-Ni angle. A 3-J-model was demanded to fit the temperature dependence of the magnetic susceptibility. Magnetostructural correlations among all known NiII tetranuclear clusters comprising a [Ni4O4] cubane core have also been performed.
Abstract
In the present work, we report the hydrothermal synthesis and detailed characterization of a series of ultramicroporous, luminescent, multivariate metal–organic frameworks (MOFs) with the general formula [Al(OH)(IATP)1−x(NH2-BDC)x], constructed from two different dicarboxylate ligands, H2IATP = 2-(((1H-imidazol-4-yl)methyl)amino) terephthalic acid and NH2-H2BDC = 2-aminoterephthalic acid. Structural analysis, using both powder X-ray diffraction (PXRD) and microcrystal electron diffraction (MicroED), confirms that the reported MTV-Al-MOFs are topological analogues of the related NH2-MIL-53(Al) MOF. Introducing ultramicroporosity (∼5.2 Å) together with bulky imidazole functional groups significantly enhances the chemical stability of these MOFs under acidic conditions, compared to NH2-MIL-53(Al). It also imparts interesting carbon dioxide physisorption properties, with MTV-Al-MOF-4 [Al(OH)(IATP)0.39(NH2-BDC)0.61] demonstrating a maximum CO2 adsorption capacity of 2.33 mmol g−1 at 273 K and a selectivity CO2/N2 of ∼76 at the same temperature (273 K) and low-pressure limit. Among the series, MTV-Al-MOF-4 exhibits the most promising combination of chemical stability and Cr(VI) sorption capacity. This material was extensively studied for eliminating Cr2O72− anions from acidic aqueous solutions, indicating high sorption capacity (174 mg Cr2O72− per g), rapid uptake kinetics (equilibrium reached within 5 minutes), and decent selectivity against competing anions such as Cl−, NO3−, and SO42−. For practical applications in wastewater treatment, a MOF-calcium alginate (CA) composite material was fabricated in beads and effectively removed Cr(VI) from diluted electroplating waste under dynamic flow conditions. Finally, all MTV-Al-MOFs displayed fluorescence with quantum yields in the range of 4.64–9.53%, while Cr(VI)-loaded MTV-Al-MOF-4 shows a pronounced reduction in fluorescence quantum yield by a factor of four, thereby confirming the presence of ligand-sorbent interactions leading to photoinduced energy and/or electron transfer processes. Overall, this study highlights a versatile approach to designing multifunctional MOFs with potential applications in environmental remediation, industrial wastewater treatment, luminescence, and gas separation.
Abstract
New Ce(IV) metal–organic frameworks (MOFs) are reported based on the aliphatic linker butane-1,2,3,4-tetracarboxylic acid (H4BTCA). These materials were prepared using an eco-friendly aqueous-based synthesis, and their structures were elucidated through microcrystal electron diffraction (MicroED) and powder X-ray diffraction (PXRD). They feature an 8-coordinated net with a bcu topology, rarely observed for Ce(IV) MOFs. Interconversion between the MOFs was easily achieved via treatment with basic or formic acid solutions, revealing the breathing of the MOFs’ frameworks. The MOFs were investigated for their ability to sorb Pb2+ under both batch and continuous flow conditions. The results showed relatively high Pb2+ sorption capacities (up to 254 mg Pb per g) and an exceptional capability for the rapid removal (in less than 10 min of MOF–solution contact) of Pb2+ from low initial concentrations of complex solutions and real-world water samples. Notably, the materials in the form of calcium alginate-based beads used as a stationary phase in a column (along with sea sand) demonstrate a remarkable ability to capture Pb2+ under continuous flow, showing decent removal capacities, excellent regeneration efficiency, and reusability. The mechanism of the Pb2+ sorption process was determined via experimental data, indicating strong interactions of the MOF's functional groups (carboxylate and hydroxide groups) with the Pb2+ ions.
2023
Abstract
Estimation of glucose (GLU) levels in the human organism is very important in the diagnosis and monitoring of diabetes. Scientific advances in nanomaterials have led to the construction of new generations of enzymatic-free GLU sensors. In this work, an innovative 3D-printed device modified with a water-stable and non-toxic metal–organic framework of iron (Fe(II)-MOF), which serves as a nanozyme, has been developed for the voltammetric determination of GLU in artificial sweat. In contrast to existing MOF-based GLU sensors which exhibit electrocatalytic activity for the oxidation of GLU in alkaline media, the nanozyme Fe(II)-MOF/3D-printed device can operate in the acidic epidermal sweat environment. The enzymatic-free GLU sensor is composed of a 3-electrode 3D-printed device with the MOF nanozyme immobilized on the surface of the working electrode. GLU sensing is conducted by differential pulse voltammetry without interference from other co-existing metabolites in artificial sweat. The response is based on the oxidation of glucose to gluconolactone, induced by the redox activity of the Fe-centers of the MOF. GLU gives rise to an easily detectable and well-defined voltammetric peak at about − 1.2 V and the limit of detection is 17.6 μmol L-1. The synergy of a nanozyme with 3D printing technology results in an advanced, sensitive, and low-cost sensor, paving the way for on-skin applications.
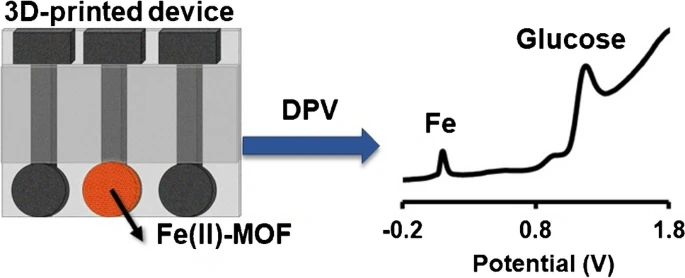
Abstract
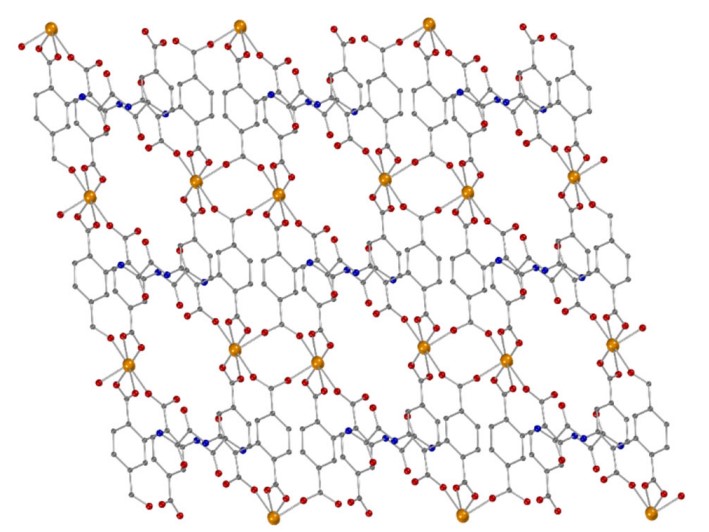
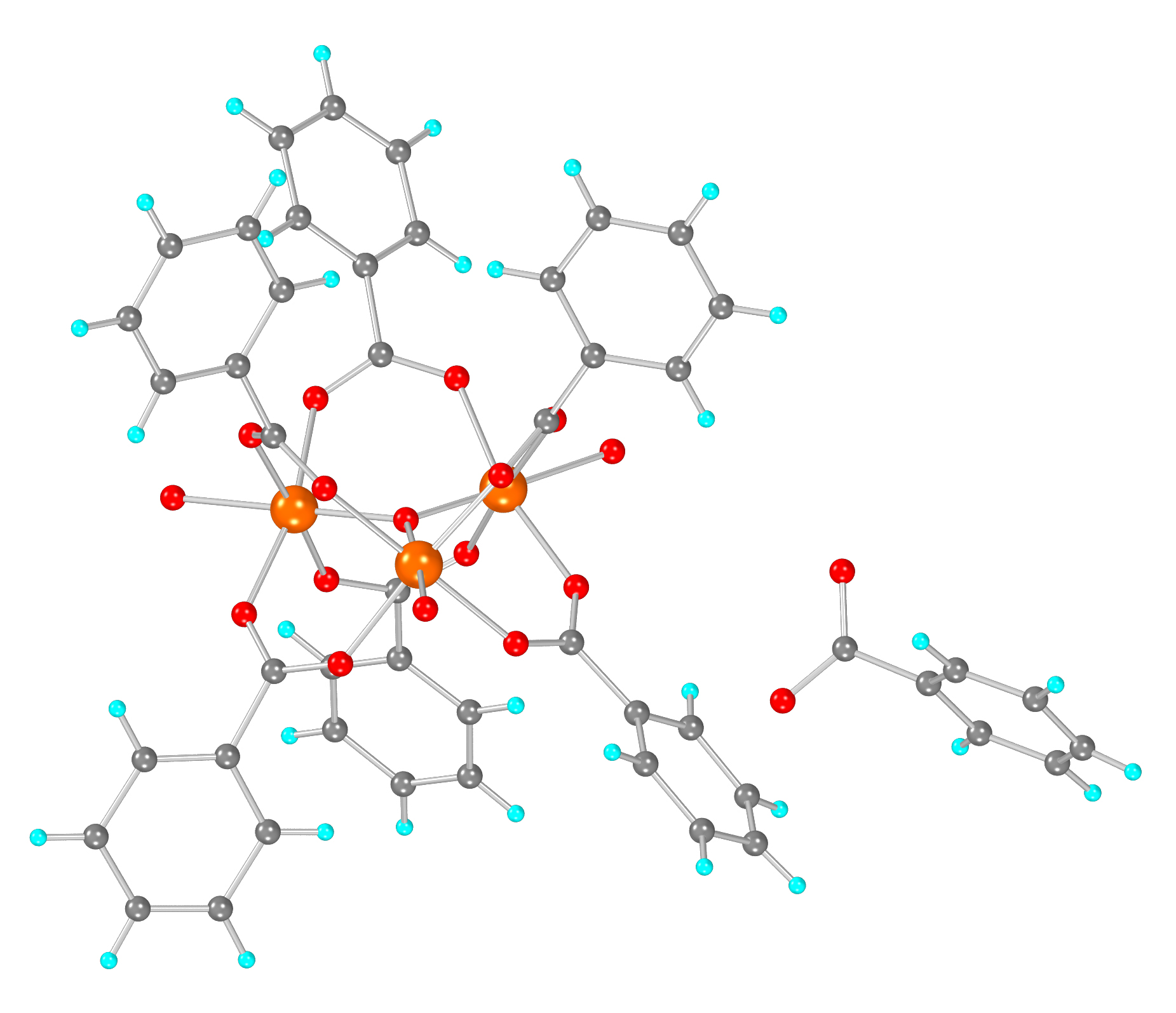
In an attempt to expand the coordination chemistry of N,N’-bis(2,4-dicarboxyphenyl)-oxalamide (H6L) ligand, we isolated and structurally characterized two new Fe(II) Metal-Organic Frameworks (MOFs), namely [Fe2(H2L)(H2O)5] (3D-Fe-MOF) and [Fe(H4L)(H2O)2]∙2H2O, (2D-Fe-MOF) by carefully adjusting the reaction conditions to achieve the optimal degree of deprotonation of the bridging ligand. Both MOFs were found stable in water, as evidenced by powder X-ray diffraction data and their ability to sorb glucose (GLU) from either an aqueous solution or artificial sweat was investigated only to show negligible sorption. Α graphite paste sensor (GPE) using the 3D-Fe-MOF as a modifier was fabricated. Τhe 3D-Fe-MOF modified GPE was assessed for non-enzymatic GLU detection in aqueous solution at pH 6 via differential pulse voltammetry and the preliminary results were discussed.
2022
Abstract
In this work, a 3D printed sensor modified with a water-stable complex of Fe(III) basic benzoate is presented for the voltammetric detection of glucose (GLU) in acidic epidermal skin conditions. The GLU sensor was produced by the drop-casting of Fe(III)-cluster ethanolic mixture on the surface of a 3D printed electrode fabricated by a carbon black loaded polylactic acid filament. The oxidation of GLU was electrocatalyzed by Fe(III), which was electrochemically generated in-situ by the Fe(III)-cluster precursor. The GLU determination was carried out by differential pulse voltammetry without the interference from common electroactive metabolites presented in sweat (such as urea, uric acid, and lactic acid), offering a limit of detection of 4.3 μmol L−1. The exceptional electrochemical performance of [Fe3O(PhCO2)6(H2O)3]∙PhCO2 combined with 3D printing technology forms an innovative and low-cost enzyme-free sensor suitable for noninvasive applications, opening the way for integrated 3D printed wearable biodevices.
Abstract
In this work, a new type of graphite paste electrode (GPE) modified with a water-stable Cu(II)-complex is described as new candidate for the voltammetric determination of glucose (GLU) and uric acid (UA) in sweat. The oxidation of CLU and UA was electrocatalyzed by Cu(II) which was electrochemically generated in-situ from the Cu(II)-complex precursor. Three in-house synthesized water-insoluble Cu(II)-complexes {[Cu(PhCOO)(H2O)2]∙PhCOO·H2O}n, [Cu(Et-saoH)2] and [Cu(Me-saoH)2] (where, Et-saoH2: 2-hydroxypropiophenone oxime, Me-saoH2: 2-hydroxyethanone oxime) were synthesized and compared with conventional copper oxides (CuO and Cu2O) as electrode modifiers for the voltammetric detection of GLU and UA in acidic media. The {[Cu(PhCOO)(H2O)2]∙PhCOO·H2O}n exhibited the most favorable electrochemical performance and the determination of both biomarkers was carried out without interference from common electroactive metabolites presented in artificial sweat, offering low limits of detection (5 μmol L−1 GLU and 4.6 μmol L−1 UA). The results confirm that {[Cu(PhCOO)(H2O)2]∙PhCOO·H2O}n-modified GPE is a promising non-enzymatic sensor for the simultaneous determination of GLU and UA in sweat.
Abstract
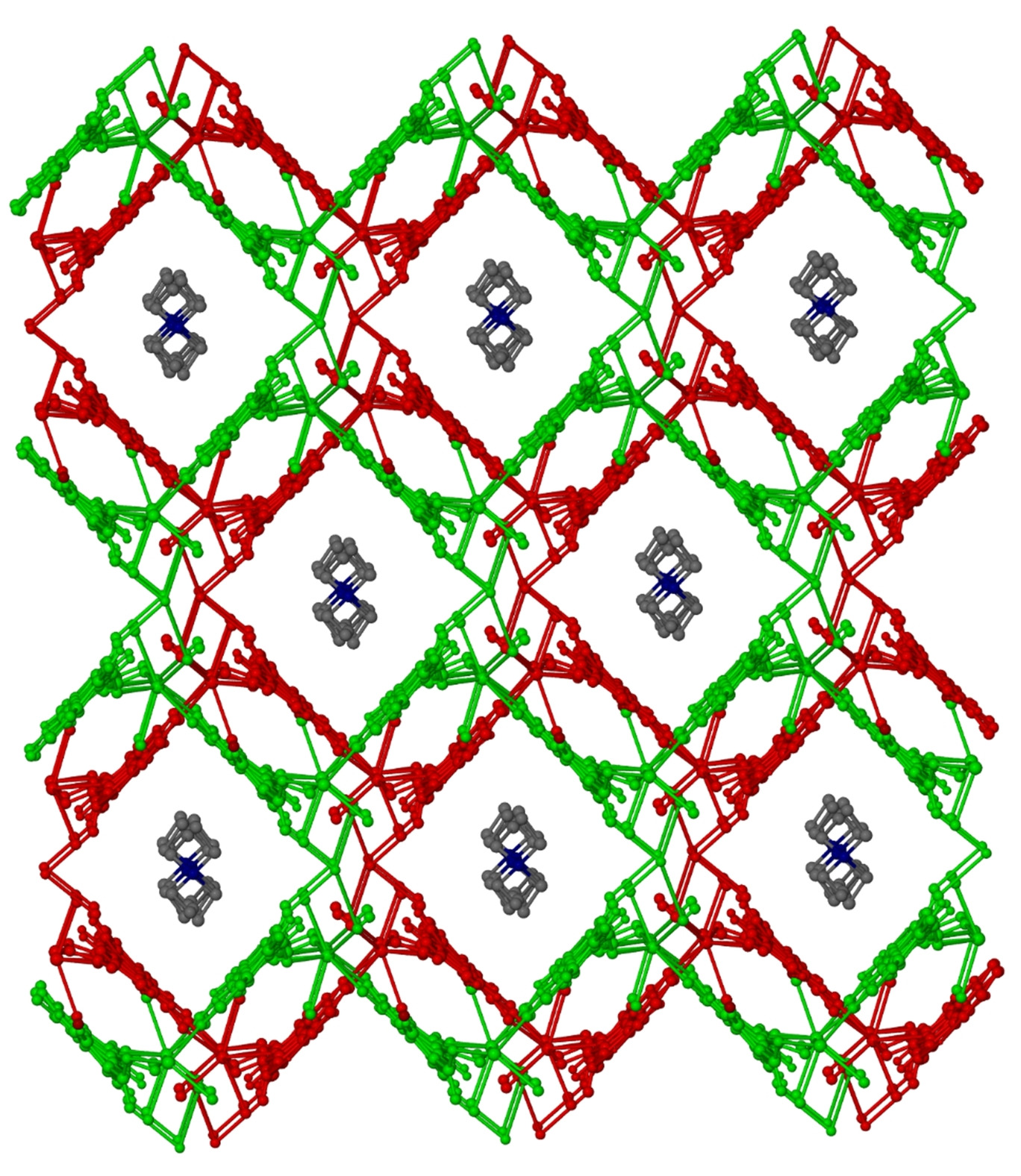
Employment of N,N'-bis(2-hydroxy,4-carboxyphenyl)-oxalamide (H 6 L) in Cu 2+ chemistry afforded the mononuclear complex (Et 4 N) 4 [CuL]∙13H 2 O ( A ) which comprises a square planar [CuL] 4– complex with several O donor atoms in its periphery. The 1:1 reaction between complex A and Ln(NO 3 ) 3 ∙xH 2 O (Ln = La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er and Yb, x = 6 or 9) in 1:4 mixture of ethanol/water in the presence of excess of KCl yielded two families of isomorphous 3d/4f coordination polymers, namely (Et 4 N) 0.5 [K 0.5 (H 2 O)Ln(H 2 O) 4 (CuL)]∙3H 2 O [Ln = La( 1 ), Ce( 2 ), Pr( 3 ), Nd( 4 ), Sm( 5 ), Eu( 6 ) and Gd( 7 )] and [K(H 2 O)Ln(H 2 O) 4 (CuL)]∙6H 2 O [Ln = Tb( 8 ), Dy( 9 ), Ho( 10 ), Er( 11 ) and Yb( 12 )]. The crystal structures of 2 - 4 revealed the presence of 3D coordination polymers while the crystal structure of 9 the presence of a 2D coordination polymer. In both 3d/4f families, complex A retains its original structure and serves as a “metallo-ligand”. The magnetic properties of 2 - 4 , 7 - 9 , 11 and 12 are discussed.
2021
Abstract
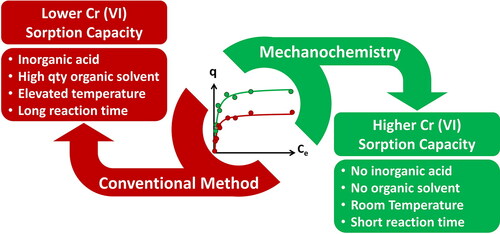
Sorption of hexavalent chromium has become a growing global concern due to its high aqueous solubility and good mobility in underground environments. Among other Metal-Organic Frameworks (MOFs), water-stable Zr-MOFs have been recently investigated for the efficient decontamination of aqueous solution from Cr(VI). Herein, Zr-benzene dicarboxylate (UiO-66) and its amino derivative, Zr-NH2-benzene dicarboxylate (UiO-66-NH2), were prepared via a facile, benign mechanochemical method (Liquid Assisted Grinding, LAG) and for the first time applied to sorption studies for Cr(VI) removal. Through the comparative examination of the mechanochemically isolated UiO-66 (LAG) and UiO-66-NH2 (LAG) with the same Zr-MOFs made via conventional solvothermal synthesis (solvothermal, SOL) UiO-66 (SOL) and UiO-66-NH2 (SOL), we demonstrate better performance of the former towards Cr(VI) sorption. The Cr(VI) sorption kinetics for all compounds were well described by the pseudo second-order kinetic model, while the calculated sorption capacities of UiO-66 (LAG) and UiO-66-NH2 (LAG) for Cr(VI) (at pH 3) by the Langmuir model were 28.8 and 36.6 mg/g, respectively, overperforming those of UiO-66 (SOL) and UiO-66-NH2 (SOL), which were found to be 24.9 mg/g and 26.1 mg/g, respectively. Our findings point out that the increased missing-linker defects, generated through milling, boost Cr(VI) sorption. In addition, this work opens the perspective for the implementation of mechanochemical synthesis in order to examine the performance of other known MOF sorbents towards water remediation applications.
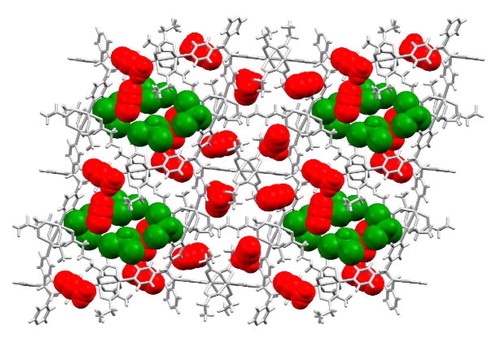
Abstract
A structural study of two-dimensional inverted metal–organic frameworks demonstrates that the interior cavities of the framework structures can be systematically modified by changing the organic anion of a Cu(II)-paddlewheel unit. Changing the anion allows modifications to the shapes and sizes of the cavities in a series of isoreticular frameworks. The construction of the frameworks is based on the application of a tetrafunctional organic cyclobutane ligand synthesized in the organic solid state.
Abstract
Employment of N,N′-bis(2-dicarboxyphenyl)-oxalamide (H4L) in the M2+ chemistry (M = Mg, Mn, Co, Ni, and Zn) afforded five 2-D coordination polymers and one 1-D coordination polymer. The crystal structures of all complexes have been determined by single-crystal X-ray crystallography. The five 2-D polymers with the general formula [M(H2L)(H2O)2] n (M = Mg 1, Mn 2, Co 3, Ni 4, Zn 6) are based on the di-anion of the oxalamide ligand (H2L2–) while the poorly diffracting complex 5, {[Ni2(L)(H2O)3]·(H2O)2} n , is based on the tetra-anion of the oxalamide ligand (L4–). The magnetic properties of 2–4 revealed weak antiferromagnetic interactions between the spin carriers.
2020
Abstract
Alkaline earth metal ion organic frameworks (AEMOFs) represent a relatively underexplored subcategory of metal–organic frameworks (MOFs). In this contribution, we present the synthesis and structural study of the new MOFs 1–8 based on the alkaline earth ions Mg2+, Ca2+, Sr2+ and Ba2+ and the amino substituted bridging ligands 4-aminonaphthalene-2,6-dicarboxylate (ANDC2−) and 4,8-diaminonaphthalene-2,6-dicarboxylate (DANDC2−). Compounds 1, 5, 6, 7 and 8 constitute rare examples of three-dimensional MOFs which feature square planar M4 secondary building units (SBUs) surrounded by eight bridging ditopic ligands. The underlying topology of MOFs 1, 5, 7 and 8 conforms to the 4-c pcb net which can be simplified to the 8-c bcu net, while 6 adopts the 4-c lta net which simplifies to the 8-c reo net. To the best of our knowledge these are the first examples of MOFs of their structural types formed by linear dicarboxylates instead of trigonal tricarboxylates or tetrahedral tetracarboxylates. Compounds 2, 3 and 4 also feature three dimensional networks with linear rod-shaped SBUs with the Ba2+ MOF 3 displaying an sra rod-net and MOFs 2 and 4 showing very complex rod-nets with so far unique topologies. Fluorescence studies revealed that the free ligands exhibit strong blue-green emission displaying considerable positive solvatochromism thereby pointing towards charge transfer excited states involving the shift of electron density from the amino groups to the aromatic core. Correspondingly, the MOFs display ligand based fluorescence with small differences in emission maxima possibly attributable to the difference in the charge density of the metal ions combined with the different environments around ligands in the crystal structures.
Abstract
In this work, a voltammetric method based on a metal organic framework (Ca-MOF)-modified carbon paste electrode for lead determination was developed. The MOF-based electrode was packed in a new type of 3D-printed syringe-type integrated device, which was entirely fabricated by a dual extruder 3D printer. After optimization of the operational parameters, a limit of detection of 0.26 µg L−1 Pb(II) was achieved, which is lower than that of existing MOF-based lead sensors. The device was used for Pb(II) determination in fish feed and bottled water samples with high accuracy and reliability. The proposed sensor is suitable for on-site analyses and provides a low-cost integrated transducer for the ultrasensitive routine detection of lead in practical applications.
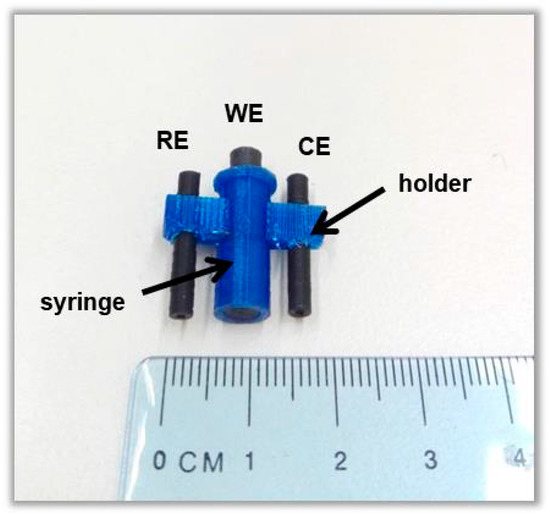
Abstract
This work combines, for the first time, 3D-printing technology and a highly efficient metal organic framework (Ca-MOF) as an electrode modifier to produce a novel fully integrated lab-in-a-syringe device for the sensitive determination of Hg(II) by anodic stripping voltammetry. The specific Ca-MOF ([Ca(H4L)(DMA)2]·2DMA where H6L is the N,N’-bis(2,4-dicarboxyphenyl)-oxalamide and DMA is the N,N-dimethylacetamide) shows an exceptional Hg(II) sorption capability over a wide pH range and its mechanism is elucidated via spectroscopic and X-ray diffraction studies. The voltammetric lab-in-a-syringe device is fabricated through a single-step process using a dual extruder 3D printer and is composed of a vessel integrating two thermoplastic conductive electrodes (serving as the counter and pseudo-reference electrodes) and of a small detachable 3D-printed syringe loaded with a graphite paste/Ca-MOF mixture (which serves as the working electrode). After optimization of the fabrication and operational variables, a limit of detection of 0.6 μg L−1 Hg(II) was achieved, which is comparable or lower than that of existing sensors (plastic 3D-printed, gold and MOF-based electrodes). The adoption of 3D printing technology in combination with the highly efficient Ca-MOF enables the fabrication of a simple, low-cost and sensitive electrochemical sensor for Hg(II), which is suitable for on-site applications.
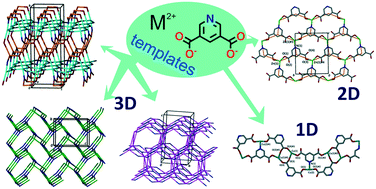
Abstract
A series of new compounds prepared from reactions of pyridine-3,5-dicarboxylic acid (PDCH2), bipositive d-metal ions (Cd2+, Zn2+, Co2+ and Cu2+) and various template molecules [1,3-propanediol (pdH2), diethanolamine (deaH3), N-phenyldiethanolamine (phdeaH2), triethanolamine (teoaH3), 2-hydroxymethylpyridine (2hmpH), 3-hydroxymethylpyridine (3hmpH), 3-hydroxypropylpyridine (3hppH), and 4-hydroxymethylpyridine (4hmpH)] are reported. Among them, [[(CH3)2NH2]2[Cd2(PDC)3]]n·4nDMF·6nH2O, [1], [Mn(PDC)(DMF)]n, [2], [Mn3(PDC)2(INA)2(DMF)1.5(H2O)0.5]n·nDMF·2nH2O, [3], and [Zn(PDC)(NMP)]n·nH2O, [4], are 3D coordination polymers, [Zn(PDC)(H2O)(DMF)]n, [5], [Zn(PDC)(3hmpH)]n·nDMF·0.5nH2O, [6], and [Co(PDC)(3hmpH)2]n·0.25nDMF, [8], have 2D polymeric structures, while [Zn(PDC)(2hmpH)2]2·2DMF, [7], is a 0D binuclear complex, and [Cu(PDC)(3hmpH)2]n·0.5nDMF·1.5nH2O, [9], is a chain polymer. Topological analysis reveals that some of the compounds display unusual structural architectures and two of them, in particular [1] and [3], present new topological types. The effect of the use of amino alcohol/polyol templates on the formation of the new compounds is also discussed in detail. The thermal stability of the prepared complexes was also studied. This study represents the initial systematic investigation on the use of a combination of polytopic nitrogen and oxygen-donor ligands and amino- or pyridine-alcohol templates for the synthesis of new MOFs.
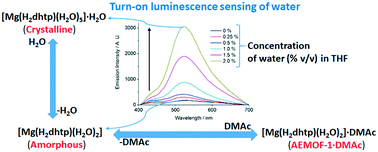
Abstract
In this contribution we build upon our previous work on the MOF [Mg(H2dhtp)(H2O)2]·DMAc (AEMOF-1·DMAc) and its activated dry version AEMOF-1′ which has been shown to exhibit excellent luminescence sensing properties towards water in organic solvents. We demonstrate through combined structural and photophysical studies that the observed changes in the fluorescence properties of AEMOF-1′ upon hydration arise from a structural transformation to the mononuclear complex [Mg(H2dhtp)(H2O)5]·H2O (H4dhtp = 2,5-dihydroxyterepthalic acid) (1). In the latter complex, excited state intramolecular proton transfer (ESIPT) is strongly favoured thereby leading to enhanced and red shifted emission in comparison to AEMOF-1·DMAc. Powder X-ray diffraction measurements confirmed that complex 1 is identical to the hydrated form of AEMOF-1·DMAc. As in the case of AEMOF-1′, the dry form of complex 1 (1′) is also an effective sensor for the determination of traces of water in tetrahydrofuran (THF). This work demonstrates that the same chromophore may exhibit very different emission properties when it exists in different chemical environments and that these transformations may be controlled and utilized in water sensing applications.
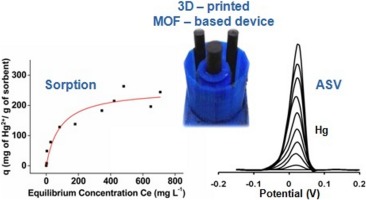
2019
Abstract
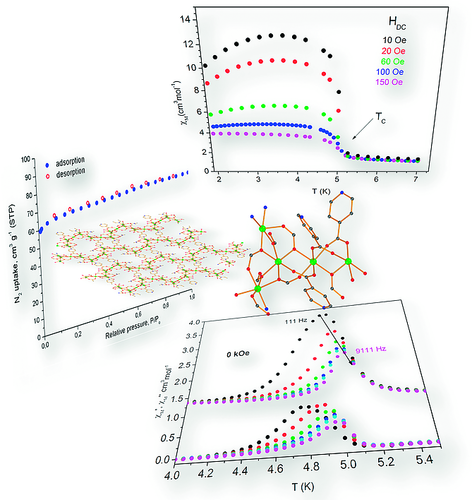
The synthesis and characterization of {[Co5(INA)3(HCOO)7(DMF)(H2O)]·2DMF·4H2O}∞ (INA– = the anion of isonicotinic acid) denoted as Co5‐INA·2DMF·4H2O is reported. The compound was isolated from the reaction of Co(NO3)2·6H2O with HINA in the presence of HCOOH in N,N‐dimethylformamide (DMF) at 100 °C. It exhibits a rigid 3‐D porous structure based on Co5 repeating units which are linked through carboxylate ligands (from HCOO– and INA–) to form an infinite rod‐shaped secondary building unit (SBU). The rods are connected through isonicotinate ligands to produce a unique (3,3,4,4,4‐coordinated) 5‐nodal 3D network with point symbol (4.5.6.72.9)(4.5.62.7.8)(4.5.7)2(5.62.73). Gas sorption studies revealed that Co5‐INA displays a microporous structure with BET area 264.5 m2 g–1 and CO2 uptake of 1.96/1.29 mmol·g–1 at 273/298 K (1 bar), respectively. Magnetism studies revealed that Co5‐INA displays spin canting behavior with a spin glass relaxation process at ca. 5.0 K.
A MOF denoted as Co5‐INA based on the isonicotinate ligand and consisting of a novel Co5 repeating unit and an unprecedented zig‐zag rod secondary building unit is presented. Co5‐INA exhibits a rigid 3‐D porous structure and a magnetic phase transition at ca. 5. 0 K.
Abstract
Voltammetric analytical techniques combine exceptional sensitivity, low cost, portability and capability for simultaneous determination of multiple analytes. The sensitivity of voltammetric analysis is largely determined by the efficiency of the working electrode. Electrodes modified with metal organic frameworks (MOFs) seem particularly promising for use in the analysis of a series of important inorganic and organic analytes. Nevertheless, research on chemically modified electrodes with MOFs is still in its infancy. In this critical review, we present the current status of research related to MOF-modified electrodes highlighting the respective MOF-modified electrodes which are based on MOFs that show exceptional chemical stability or/and sorption capability towards the targeted analytes. We also provide perspectives for future research aiming at motivating additional scientists to be involved in this exciting field of MOF-based electroanalytical sensors.
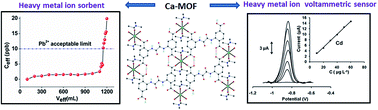
Abstract
We recently discovered that a Ca2+ two-dimensional framework, namely [Ca(H4L)(DMA)2]·2DMA (Ca-MOF), was capable of exchanging the Ca2+ ions with Cu2+ almost quantitatively in a matter of seconds in aqueous solution. Herein, we report that Ca-MOF exhibits the capability of both removal and voltammetric determination of heavy metal ions in aqueous media. Ca-MOF shows one of the highest Pb2+ sorption capacities (∼522 mg g−1) reported for MOFs. More importantly, a column filled with Ca-MOF (1% wt) particles dispersed in silica sand (99% wt) can quantitatively remove traces of Pb2+ (∼100 ppb) from a relatively large volume of a wastewater simulant solution (containing a large excess of competitive ions). Ca-MOF is also highly efficient for sorption of Cd2+, Ni2+ and Zn2+, even in the presence of several competitive cations. Actually, the Cd2+ sorption capacity (∼220 mg g−1) of Ca-MOF is one of the largest reported for MOFs. Furthermore, detailed Ni2+ and Zn2+ sorption studies of MOFs have not been described prior to this work. The mechanism of the M2+ (M2+ = Pb2+, Cd2+, Ni2+, Zn2+) exchange process was elucidated based on a series of spectroscopic, analytical and X-ray diffraction methods. In addition, a simple ready-to-use electrochemical sensor based on modified graphite paste with Ca-MOF was fabricated and successfully utilized for the determination of Pb2+, Cd2+, Cu2+ and Zn2+ at μg L−1 levels in aqueous solutions by anodic stripping voltammetry (ASV). Overall, this work demonstrates, for the first time, a dual function of a MOF as a sorbent and as an electrochemical sensor for heavy metal ions, thus opening a new window for materials with application in both environmental remediation and monitoring.
2018
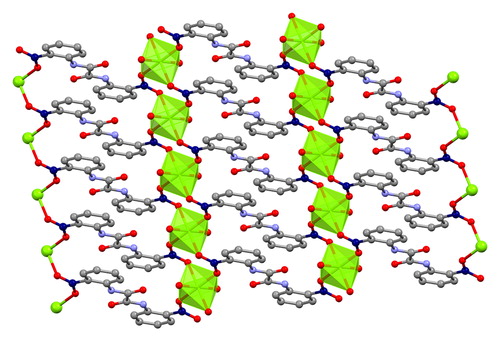
Abstract
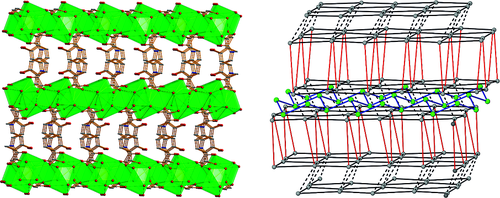
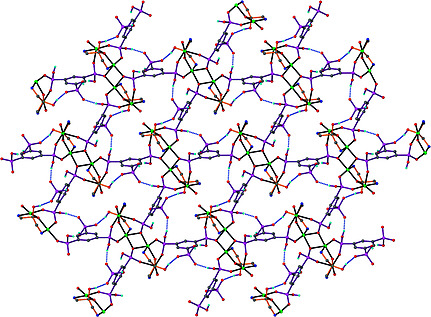
The use of 4,4′‐[oxalylbis(azanediyl)]bis(2‐hydroxybenzoic acid) (H6L1) in the Ba2+ chemistry has afforded a 3D polymer, namely [Ba(H2L2)(H2O)]n (1), which is based on H2L22– anions derived by the in‐situ metal‐assisted transformation of H6L1. The neutral H4L2 [4‐(carboxyformamido)‐2‐hydroxybenzoic acid] ligand was isolated from 1 and characterized by spectroscopic methods. Polymer 1 is based on edge and face‐sharing BaO10 polyhedra which create an inorganic layer pillared to the third dimension by the organic ligands and has been classified as an I2O1 framework. The topological analysis of 1 provided an opportunity to introduce a method for the deconstruction of I2O1 frameworks by adopting principles applied in the deconstruction of Metal‐Organic‐Frameworks (MOFs) with rod Secondary Building Units (SBUs). A detailed discussion and insights for the proper use of the ImOn notation which finds application in describing the dimensionality in MOFs, is also provided.
The 3D polymer [Ba(H2L2)(H2O)]n (1), (H2L22– derived from an in‐situ metal‐assisted transformation) is reported. H4L2 was isolated from 1 and characterized. 1 is based on edge and face‐sharing BaO10 polyhedra which create an inorganic layer pillared to the third dimension by the H2L22– and classified as an I2O1 framework. A detailed discussion and insights for the proper use of the ImOn notation is provided. A reliable method for the deconstruction of I2O1 frameworks is also introduced.
Abstract
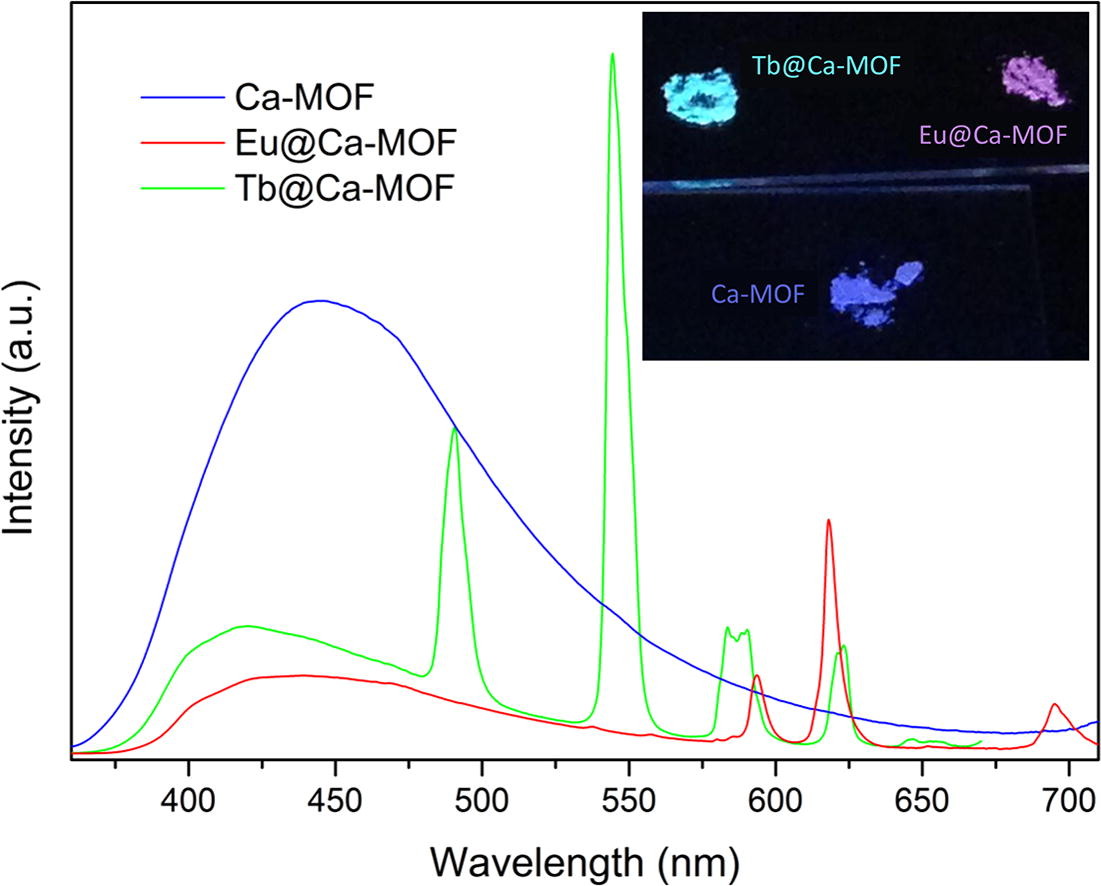
Very recently, we discovered that the two-dimensional framework, namely [Ca(H4L)(DMA)2]·2DMA (Ca-MOF) was capable of exchanging the Ca2+ ions by Cu2+ almost quantitatively in a matter of seconds in aqueous solution and that enabled us to utilized the Ca-MOF as an ion-exchange material with potential applications in water treatment and/or recovery of valuable metals. To this end, we turned our attention toward exchanging the Ca2+ ion by lanthanides [i.e. Tb3+ and Eu3+] in order to afford materials that emit white-light. We discovered that the Ca-MOF slowly exchanges approximately 95% of the Ca2+ ions by Tb3+/Eu3+ yielding materials that combine the initial blue emission of the Ca-MOF with the green and red emission of the Tb3+ and Eu3+, respectively, leading to materials with almost white-light emission.
A blue-light photoluminescence Ca-MOF capable of exchanging ∼95% of the Ca2+ ions by Tb3+ or Eu3+ or combinations of them yields a series of EuxTby@Ca-MOF materials that combine the initial blue emission with the green and red photoluminescence of the Tb3+ and Eu3+, respectively, leading to materials with almost white-light emission. The present work indicates that Alkaline Earth MOFs may serve as precursors for the isolation of new materials with potential applications in white light emitting diodes.
Abstract
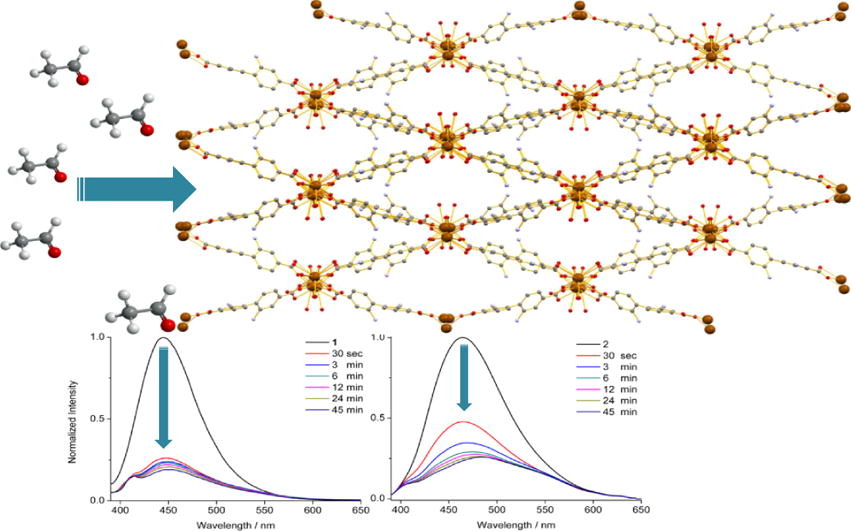
Alkaline earth metal ion organic frameworks (AEMOFs) represent a relatively underexplored subcategory of MOFs. Two new MOFs [Ca6(bpdc-(NH2)2)5(μ3-HCO2)2(H2O)2.5(DMF)0.5]·0.5H2O·2.5DMF (1) and [Sr4(bpdc-(NH2)4)(μ2-DMF)2(DMF)1/3]·2/3(DMF) (2) [H2bpdc-(NH2)2 = 2,2′-diamino-[1,1′-biphenyl]-4,4′-dicarboxylic acid); DMF = N,N-dimethylformamide] are presented here. These MOFs display structural variety with diverse topologies and new structural features. Luminescence studies revealed that both MOFs display ligand based fluorescence with small differences in emission profiles possibly attributable to the difference in charge density of the metal ions combined with the different conformation adopted by the ligand in the crystal structures of 1 and 2. Furthermore, initial sensing studies reveal that both MOFs can potentially function as fluorescent sensors for gas phase aldehydes.
In this work we present two new fluorescent MOFs based on the alkaline earth metal ions Ca2+ and Sr2+. The MOFs feature three dimensional networks which contain uncoordinated amino groups. In addition, both MOFs display bright blue fluorescence in the solid state. Their fluorescence is strongly quenched upon their interaction with gas phase aldehydes indicating that MOFs of this type may form the basis for the development of new aldehyde sensing platforms.
Abstract
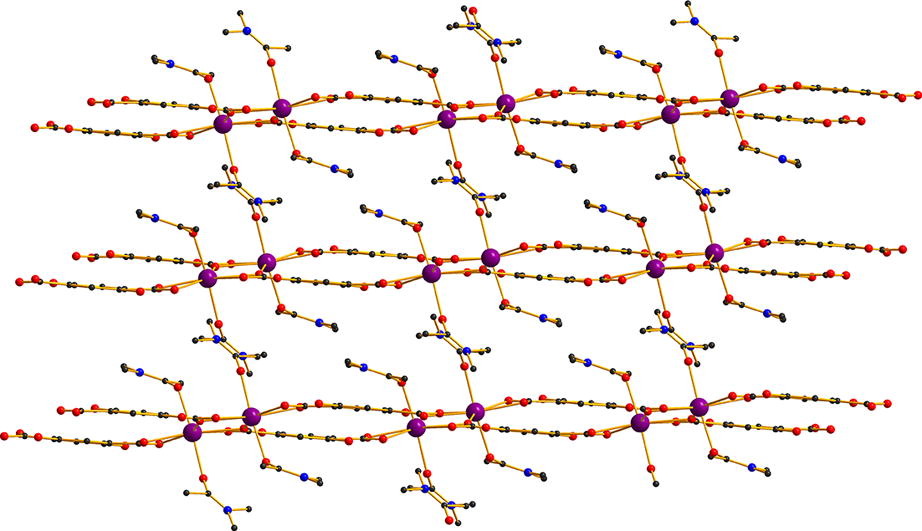
We report here a new metal ion organic framework [Cd(H2dhtp)(DMAc)2] (CdMOF-1) (H2dhtp2− = 2,5-dihydroxy-terephthalate, DMAc = N,N-dimethylacetamide). CdMOF-1 shows a 2-D structure based on interconnected dinuclear [Cd2(COO)4(DMAc)4] repeating units. It represents the first example of a 2-D Cd2+-H2dhtp2− compound and only the second M2+-H2dhtp2− MOF with a two-dimensional polymeric structure. CdMOF-1 is strongly luminescent showing intense (turquoise) emission at ca. 498 nm. Detailed investigations of the photophysical properties of the MOF were performed via UV–Vis solid state spectroscopy, steady state and time-resolved variable temperature luminescence studies.
A highly luminescent Cd2+-dihydroxyterephthalate MOF that shows a 2-D framework structure rarely seen for metal ion-H2dhtp2− MOFs is reported, along with detailed investigations of its photophysical properties.
Abstract
The ever-increasing need to determine and monitor the chemical constituents of the constantly evolving environment has led the global scientific community to invest considerable research effort in the development of efficient and user-friendly chemical sensors. The development of improved chemical sensors largely depends on the synthesis of novel materials with the ability to transform a molecular recognition event into a readable signal. Among the various types of sensory materials, those where analyte detection is based on the change of a luminescence signal are gaining increasing attention due to the extremely high sensitivities which can be achieved in combination with new technological advances enabling the integration of optical detection systems in small, portable and easy to use devices. In this critical review we approach the emerging field of sensory materials based on luminescent metal–organic frameworks (LMOFs) by beginning with a survey of the general principles of luminescence-based sensing. In particular, after a brief overview, we first focus on the working principles and successes of well established sensory materials based on small molecules and conjugated polymers. Subsequently, we concentrate on the special features of LMOFs which make them promising sensory materials and we discuss best practices which researchers in the field should follow in order to prove the sensing ability of LMOFs and avoid common misconceptions and errors. We continue with presenting selected examples of LMOF-based sensors for nitroaromatics, humidity and heavy metal ions from the recent literature and we conclude with a summary of the state-of-the-art of LMOF sensors. Finally, we propose some directions for future research on LMOF sensors.
Abstract
Enlargement of a self-assembled metal–organic rhomboid is achieved via the organic solid state. The solid-state synthesis of an elongated organic ligand was achieved by a template directed [2 + 2] photodimerization in a cocrystal. Initial cocrystals obtained of resorcinol template and reactant alkene afforded a 1:2 cocrystal with the alkene in a stacked yet photostable geometry. Cocrystallization performed in the presence of excess template resulted in a 3:2 cocrystal composed of novel discrete 10-component hydrogen-bonded “superassemblies” wherein the alkenes undergo a head-to-head [2 + 2] photodimerization. Isolation and reaction of elongated photoproduct with Cu(II) ions afforded a metal–organic rhomboid of nanoscale dimensions that hosts small molecules in the solid state as guests.
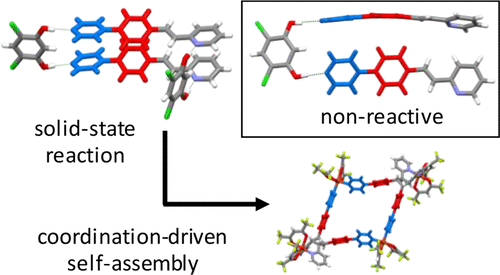
Abstract
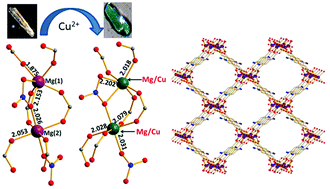
In our effort to develop new synthetic strategies for the isolation of new phosphonate frameworks, we exploited the possibility of simultaneously utilizing benzene‐1,3,5‐triphosphonic acid (H6btp) and di‐2‐pyridyl ketone (py2CO), which is a major player in the field of polynuclear metal complexes. This ligand blend in CuII chemistry afforded a 2D framework comprising tetranuclear [CuII4] secondary building units. A thorough investigation of the CuII/di‐2‐pyridyl ketone/benzene‐1,3,5‐triphosphonic acid reaction system also revealed a hydrogen‐bonded framework. The structure of the latter comprises the cationic [Cu{py2C(OH)2}2]2+ complex [py2C(OH)2 is the hydrate of py2CO], the dianion of benzene‐1,3,5‐triphosphonic acid (H4btp2–) and water solvates. The magnetic properties of the coordination polymer were rationalized on the basis of magnetically isolated [CuII4] units dominated by antiferromagnetic interactions.
The simultaneous use of the metal‐nucleating ligand di‐2‐pyridyl ketone and the bridging benzene‐1,3,5‐triphosphonic acid ligand in CuII chemistry afforded a hydrogen‐bonded framework and a 2D coordination polymer comprising tetranuclear SBUs. The magnetic properties of the product were rationalized on the basis of magnetically isolated [CuII4] units dominated by antiferromagnetic interactions.
2017
Abstract
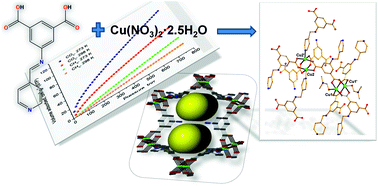
A new Ca2+ two-dimensional framework, namely [Ca(H4L)(DMA)2]·2DMA (Ca-MOF) was obtained from the initial use of N,N′-bis(2,4-dicarboxyphenyl)-oxalamide (H6L). We discovered that this Ca-MOF is capable of exchanging the Ca2+ ions by Cu2+ almost quantitatively in a matter of seconds in aqueous solution. The highly efficient Cu2+ sorption properties exhibited by the Ca-MOF were investigated in detail via batch ion-exchange studies. In addition, the Ca-MOF was utilized as a stationary phase in an ion-exchange column for Cu2+ removal from aqueous media. Furthermore, we were able to recycle the most expensive part of the framework, i.e. the H6L, by treating the column with dilute NaOH followed by HCl. The Ca-MOF represents the first example of any MOF capable of exchanging its constituent metal ions that is shown to be a highly effective ion-exchange material, thus opening a new window in the exploitation of MOFs for ion-exchange applications.
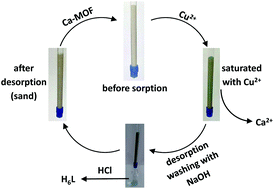
Abstract
The reactions of Al(III), Ga(III) and In(III) nitrates with 2-quinaldic acid (qaH) afforded [Al2(OH)2(qa)4]·2H2O (1), [Ga(qa)2(H2O)2]NO3 (2) and [In(qa)2(NO3)(H2O)] (3), respectively, in high yields. The crystal structures of 1, 2 and 3 have been determined by single-crystal X-ray crystallography. The structure of 1 features a di-hydroxo bridged [Al2(μ-OH)2]4+ dimer in which each Al(III) is further ligated by two bidentate chelate qa− ligands. Complexes 2 and 3 are mononuclear with the M(III) ions in octahedral environments surrounded by two bidentate chelate qa− and two H2O in 2 or one H2O and a terminal NO3− in 3. Characteristic IR as well as thermal analysis and solid-state fluorescence are discussed.
Abstract
We report herein a new alkaline earth metal ion organic framework [Mg2(NH2BDC)2(HNO3)]·9H2O (AEMOF-7), which shows a 3-D microporous structure with several unusual features, such as the rare trigonal prismatic coordination geometry of one of the crystallographically unique Mg2+ centers and the existence of a bridging HNO3 ligand. The H+ ions of the HNO3 ligand are dissociable as demonstrated via proton conductivity measurements. AEMOF-7 displays relatively high selectivity for CO2vs. CH4 and negligible N2 uptake. Interestingly, this compound was found to be capable of single-crystal-to-single-crystal (SCSC) exchange of Mg2+ by Cu2+ ions, which was observed for the first time in a MOF material. AEMOF-7 is also luminescent and its photophysical properties were investigated via solid state UV-Vis, steady-state and time-resolved luminescence studies.
Abstract
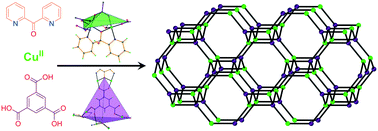
Employment of di-2-pyridyl ketone and poly-carboxylates in CuII chemistry afforded four complex hydrogen-bonded frameworks, one one-dimensional (1D), one 2D and three 3D coordination polymers. Di-2-pyridyl ketone underwent several metal-assisted transformations to yield three CuII structural units which, in combination with the poly-carboxylate anions of the trimesic, isophthalic, 5-hydroxy-isophthalic and pyromellitic acids, provided access to extended frameworks by dative or hydrogen bonds. All nine complex frameworks were realized in terms of their topological analysis. The 3D and the 2D polymers consisted of [Cu2] dimers and were found to be dominated by ferromagnetic interactions. The origin of the ferromagnetic coupling was attributed to the counter complementarity of the simultaneous alkoxo/syn,syn-carboxylate bridges within the dimers.
2016
Abstract
A new Cu2+ complex that was isolated from the initial use of 5-((pyridin-4-ylmethylene)amino)isophthalic acid (PEIPH2) in 3d metal–organic framework (MOF) chemistry is reported. Complex {[Cu3(PEIP)2(5-NH2-mBDC)(DMF)]·7DMF}∞ denoted as Cu-PEIP·7DMF was isolated from the reaction of Cu(NO3)2·2.5H2O with PEIPH2 in N,N-dimethylformamide (DMF) at 100 °C and contains both the PEIP2− ligand and its 5-NH2-mBDC2− fragment. After the structure and properties of Cu-PEIP were known an analogous complex was prepared by a rational synthetic method that involved the reaction of Cu(NO3)2·2.5H2O, 5-((pyridin-4-ylmethyl)amino)isophthalic acid (PIPH2 – the reduced analogue of PEIPH2) and 5-NH2-mBDCH2 in DMF at 100 °C. Cu-PEIP comprises two paddle-wheel [Cu2(COO)4] units and exhibits a 3D-framework with a unique trinodal underlying network and point symbol (4.52)4(42·54·64·83·92)2(52·84). This network consists of pillared kgm-a layers containing a hexagonal shaped cavity with a relatively large diameter of ∼8–9 Å surrounded by six trigonal shaped ones with a smaller diameter of ∼4–5 Å and thus resembles the structure of HKUST-1. Gas sorption studies revealed that Cu-PEIP exhibits a 1785 m2 g−1 BET area as well as high CO2 sorption capacity (4.75 mmol g−1 at 273 K) and CO2/CH4 selectivity (8.5 at zero coverage and 273 K).
2015
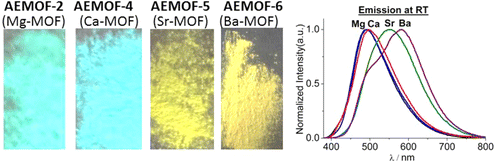
Abstract
Alkaline earth (group 2) metal ion organic frameworks (AEMOFs) represent an important subcategory of MOFs with interesting structures and physical properties. Five MOFs, namely, [Mg2(H2dhtp)2(μ-H2O)(NMP)4] (AEMOF-2), [Mg2(H2dhtp)1.5(DMAc)4]Cl·DMAc (AEMOF-3), [Ca(H2dhtp)(DMAc)2] (AEMOF-4), [Sr3(H2dhtp)3(DMAc)6]·H2O (AEMOF-5), and [Ba(H2dhtp)(DMAc)] (AEMOF-6) (H4dhtp = 2,5-dihydroxy-terepthalic acid; DMAc = N,N-dimethylacetamide; NMP = N-methylpyrrolidone), are presented herein. The reported MOFs display structural variety with diverse topologies and new structural features. Interestingly, AEMOF-6 is the first example of a Ba2+–H2dhtp2– MOF, and AEMOF-5 is only the second known Sr2+–H2dhtp2– MOF. Detailed photoluminescence studies revealed alkaline earth metal ion-dependent fluorescence properties of the materials, with the heavier alkaline earth metal ions exhibiting red-shifted emission with respect to the lighter ions at room temperature. A bathochromic shift of the emission was observed for the MOFs (mostly for AEMOF-3 and AEMOF-4) at 77 K as a result of excited state proton transfer (ESIPT), which involves an intramolecular proton transfer from a hydroxyl to an adjacent carboxylic group of the H2dhtp2– ligand. Remarkably, AEMOF-6 displays rare yellow fluorescence at room temperature, which is attractive for solid state lighting applications. To probe whether the alkaline earth metal ions are responsible for the unusual luminescence properties of the reported MOFs, the potential energy surfaces (PESs) of the ground, S0, and lowest energy excited singlet, S1, states of model complexes along the intramolecular proton transfer coordinate were calculated by DFT and TD-DFT methods.
Abstract
The development of efficient sensors for the determination of the water content in organic solvents is highly desirable for a number of chemical industries. Presented herein is a Mg2+ metal–organic framework (MOF), which exhibits the remarkable capability to rapidly detect traces of water (0.05–5 % v/v) in various organic solvents through an unusual turn‐on luminescence sensing mechanism. The extraordinary sensitivity and fast response of this MOF for water, and its reusability make it one of the most powerful water sensors known.
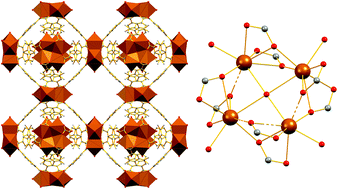
Abstract
The synthesis and characterization of {[Co9(INA)18(H2O)6]·11DMF·15H2O}∞ (Co9-INA·11DMF·15H2O) (INA– = the anion of isonicotinic acid) is reported. It exhibits a rigid 3D-porous structure with a Co9 repeating unit consisting of four [CoII2(μ-O2CR)2(μ-H2O)] subunits (two unique) linked through bridging INA– ligands to an isolated CoII ion (half unique). The [CoII2] dimers and the isolated CoII ion have assembled to create a trinodal (6,7,8)-coordinated network with point symbol (32.411.56.62)2(32.418.54.64)2(34.44.54.63). Gas sorption studies revealed that Co9-INA exhibits 910 m2 g–1 BET area, 4.2 mmol g–1 CO2 uptake at 273 K/1 bar, and 6.7 CO2/CH4 selectivity at zero coverage. Furthermore, Co9-INA displays capability for exchange of the guest solvent molecules by various organic molecules in a single-crystal to single-crystal fashion. Direct and alternating current magnetic susceptibility studies revealed the existence of dominant antiferromagnetic interactions between the Co2+ ions that result in a paramagnetic ST = 3/2 spin ground state value. Overall, this work emphasizes the potential of relatively simple and inexpensive polytopic ligands, such as isonicotic acid, to stabilize microporous MOFs with significant CO2 sorption capacity.
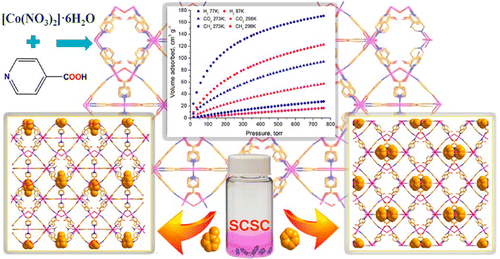
2014
Abstract
We describe hydrogen-bonded dimers of catechol that act collectively as a single template to direct an intermolecular [2+2] photocycloaddition in the solid state. The directed reactivity involves discrete, six-component hydrogen-bonded assemblies and a photoreaction that occurs stereoselectively and in quantitative yield.
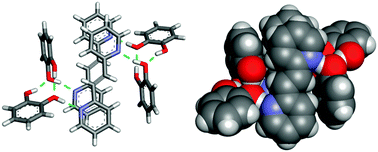
Abstract
A [CuII16] wheel was isolated serendipitously from the reaction of acetylacetone dioxime with copper(II) chloride and lanthanide ions in a reaction initially designed to produce heterometallic 3d–4f cages. The ligand has been transformed in situ to three different forms, all found within the [Cu16] wheel, with the original ligand completely absent.
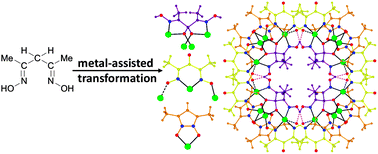
Abstract
A family of exceptionally thermally stable [Ni8] cages is reported, each being templated by a rare η3:η3:μ6-O22− species produced by dioxygen activation, where the reducing agent for the O2 reduction appears to be the ligand used in the reaction mixtures, which was found within the nickel cages in its oxidized form.
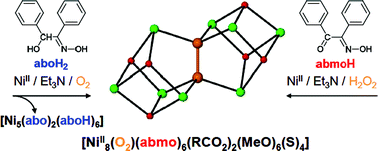
Abstract
A copper trimesate selenite compound is reported which shows a robust 3-D porous structure with unprecedented structural characteristics and represents a unique example of a metal inorganic–organic framework with considerable permanent microporosity and selective CO2 sorption properties.
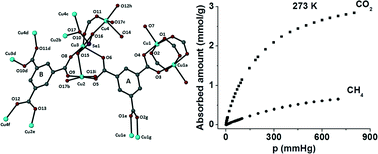
Abstract
In an attempt to employ salicylic acid (HOsalH), 2,6-dihydroxy benzoic acid {(HO)2PhCO2H}, and naphthalene-1,8-dicarboxylic acid {1,8-naph(CO2H)2} in Mn(III) salicylaldoximate chemistry as a means to alter the structural identity of the hexanucluear clusters usually obtained from this reaction system, we have isolated a family of hexanuclear Mn(III) complexes based on salicyladloxime (saoH2) and 2-hydroxy-1-naphthaldehyde oxime (naphthsaoH2). Five hexanuclear clusters, [Mn6O2(sao)6(HOsal)2(EtOH)4]·EtOH (1·EtOH), [Mn6O2(sao)6{1,8-naph(CO2Me)(CO2)}2(MeOH)6]·3MeOH (2·3MeOH), [Mn6O2(naphthsao)6{1,8-naph(CO2Et)(CO2)}2(EtOH)6] (3·2MeOH), [Mn6O2(naphthsao)6(MeCO2)2(EtOH)4]·2H2O (4·2H2O), and [Mn6O2(naphthsao)6{(HO)2PhCO2}2(EtOH)4]·4EtOH (5·4EtOH), have been synthesized and characterized by single-crystal X-ray crystallography. The magnetic properties of 3, 4, and 5 are discussed.
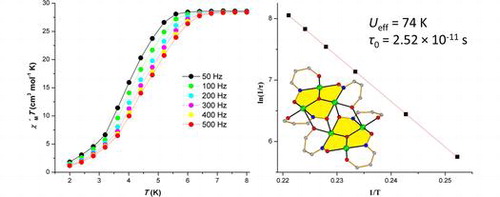
2013
Abstract
The reactions of N,N′-bis(salicylidene)propane-1,3-diamine (salpropH2) and its substituted derivatives with Ga(acac)3 afforded the complexes [Ga(acac)(salprop)]·0.5H2O (1), [Ga(acac)(5Clsalprop)] (2), [Ga(acac)(5Brsalprop)] (3), [Ga(acac)(5NO2salprop)] (4) and [Ga(acac)(5Mesalprop)] (5), in high yields. The crystal structures of all complexes have been determined by single-crystal X-ray crystallography. All complexes are mononuclear with the Ga(III) atoms being in octahedral environments surrounded by one tetradentate chelate 5Rsalprop2− ligand and one bidentate chelate acac− ligand. The free ligands exhibit photoluminescence which was found to be increased upon complex formation. The substituted derivatives of the salpropH2 ligand bearing either electron-donating methyl groups or electron-withdrawing groups, such as Cl, Br and NO2 at the fifth position of both salicylidene rings were found to modify the emission maximum of the free ligands and the complexes.
A family of mononuclear Ga(III) complexes based on N,N′-bis(salicylidene)propane-1,3-diamine (salpropH2) and its substituted derivatives has been synthesized and structurally characterized. The peripheral substitution of the salpropH2 ligand with EDGs or EWGs modulates the emission maxima of the free ligands and the complexes.
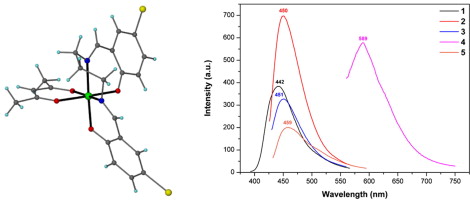
Abstract
A 1-D coordination
2012
Abstract
A new flexible Cd2+ metal organic framework (MOF), denoted as UCY-3, which is the second example of a MOF with the
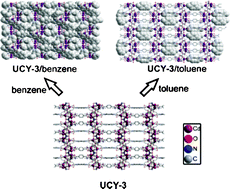
Abstract
A series of new Zn2+-trimesate (btc3-) metal organic frameworks (MOFs) has been isolated in the presence of various amino-alcohols under solvothermal conditions. Thus, the reaction of ZnCl2 with trimesic acid (H3btc) and the amino-alcohols triethanolamine (teoa), 2-(hydroxymethyl)piperidine (hmpip), N-tert-butyldiethanolamine (tbdeoa), 1,4-bis(2-hydroxyethyl)piperazine (bhep), N-methyldiethanolamine (mdeoa), or 4-(2-hydroxyethyl)morpholine (hem) in a 1.6:1:5.6 molar ratio in DMF afforded compounds (teoaH)2[Zn(btc)1.33] (MOAAF-1) (MOAAF = metal organic amino-alcohol framework), (NH2Me2)2(hmpipH)[Zn3(btc)3] (MOAAF-2), (NH2Me2)(tbdmaH)2[Zn3(btc)3] (MOAAF-3) (tbdma = N-tert-butyl-dimethylamine), (NH2Me2)(bhepH2)[Zn3(btc)3] (MOAAF-4), (NH2Me2)[Zn4(btc)3(mdeoa)2] (MOAAF-5), and (NH2Me2)[Zn4(btc)3(hem)2] (MOAAF-6), respectively. The compounds display 3D structures with relatively large cavities (4–10 Å) and high potential solvent-accessible areas (38–68% of the unit cell volumes). A number of novel structural features are revealed in the reported MOFs, such as unprecedented dinuclear [Zn2(COO)5]−1 secondary building units (SBUs) and unique network topologies (e.g., in compounds MOAAF-2, MOAAF-3, MOAAF-5, and MOAAF-6). The amino-alcohols employed played a key role for the appearance of such novel structural features in MOAAF 1–6 since they were found to act as bases responsible for the deprotonation of H3btc, templates, and chelating ligands. Specifically, most of the compounds synthesized were shown to be templated by protonated amino-alcohols that are involved in hydrogen bonding interactions with the frameworks, whereas in two cases (compounds MOAAF-5 and MOAAF-6) the amino-alcohols acted as chelating ligands affecting significantly the underline topology of the MOFs. The thermal stability and photoluminescence properties of the MOFs are also discussed. This work represents the initial systematic investigation on the use of combination of amino-alcohols and polycarboxylate ligands for the synthesis of new MOFs, demonstrating it as a powerful synthetic strategy for the isolation of novel MOFs.
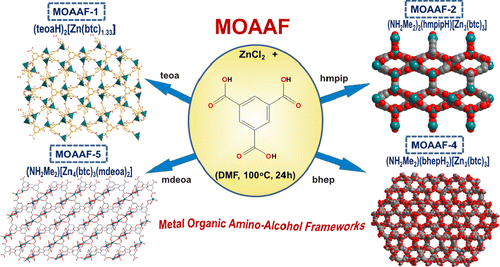
Abstract
Single-crystal-to-single-crystal (SCSC) transformations represent some of the most fascinating phenomena in chemistry. They are not only intriguing from a basic science point of view but also provide a means to modify or tune the properties of the materials via the postsynthetic introduction of suitable guest molecules or organic functional groups into their structures. Here, we describe UCY-2, a new flexible Nd3+ metal–organic framework (MOF), which exhibits a unique capability to undergo a plethora of SCSC transformations with some of them being very uncommon. These structural alterations involve the replacement of coordinating solvent molecules of UCY-2 by terminally ligating solvents and organic ligands with multiple functional groups including −OH, −SH, −NH–, and −NH2 or their combinations, chelating ligands, anions, and two different organic compounds. The SCSC coordinating solvent exchange is thus demonstrated as a powerful method for the functionalization of MOFs.
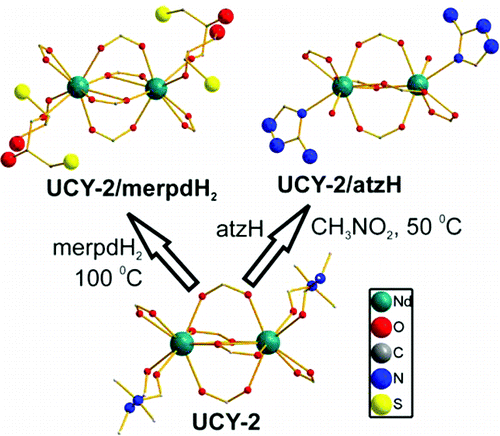
Abstract
Fine tuning the Mn/salicylaldoxime/trimesic acid reaction conditions leads to the formation of a regular 2D net held together by dative bonds and to a non-regular 2D net stabilised by both dative and hydrogen bonds. Both networks are built from [Mn6] Single-Molecule Magnets.
2011
Abstract
The initial use of a novel elongated tricarboxylic acid H3hmpib in metal–organic framework (MOF) chemistry resulted in a [Zn4O(hmpib)2] MOF (UCY-1) with pyrite topology. The compound displays a remarkably high internal surface area despite its double-interpenetrated structure as well as high CO2 uptake and selective adsorption for it over CH4.
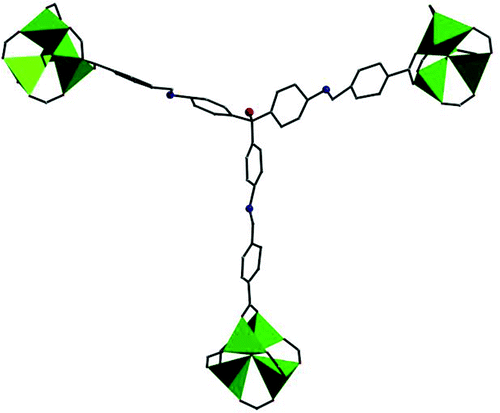
Abstract
The exploration of the NiX2/py2CO/Et3N (X = F, Cl, Br, I; py2CO =
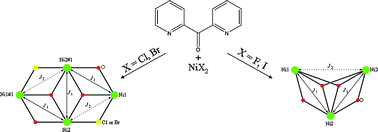
Abstract
The syntheses, structures and magnetic properties of six iron complexes stabilised with the derivatised
Abstract
A [Mn(III)9] partial supertetrahedron is a Single-Molecule Magnet (SMM) with an energy barrier to magnetisation reversal of ∼30 K and represents the first chiral SMM obtained from achiral starting materials.
2010
Abstract
N,N'-diethylurea (DEU) was employed as a ligand to form the octahedral complexes (M=Co, Ni and Zn). Compounds (1), (2), (3), and (4) have been prepared from the reactions of DEU and the appropriate hydrated metal(II) salts in EtOH in the presence of 2,2-dimethoxypropane. Crystal structure determinations demonstrate the existence of cations and (in 2–4) or (in 1) counterions. The cations in the solid state are stabilized by a pseudochelate effect due to the existence of six strong intracationic hydrogen bonds. The cations and counterions self-assemble to form hydrogen-bonded 2D architectures in 2–4 that conform to the kgd (kagome dual) network, and a 3D hydrogen-bonded rtl (rutile) network in 1. The nature of the resulting supramolecular structures is influenced by the nature of the counter-ion. The complexes were also characterized by vibrational spectroscopy (IR).
Abstract
As part of our interest into the bioinorganic chemistry of gallium, gallium(III) complexes of the azole ligands 2,1,3-benzothiadiazole (btd), 1,2,3-benzotriazole (btaH), and 1-methyl-4,5-diphenylimidazole (L) have been isolated. Reaction of btaH or btd with or resulted in the mononuclear complexes (1) and (2), respectively, while treatment of with L resulted in the anionic complex (3). All three complexes were characterized by single-crystal X-ray crystallography and IR spectroscopy, while their antiproliferative activities were investigated against a series of human and mouse cancer cell lines.
Abstract
Having established that molecules with general formulae [MnIII6O2(R-sao)6(O2CR)2(L)4–6] ([Mn6]) and [MnIII3O(R-sao)3(X)(L)3] ([
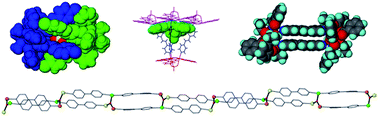
Abstract
We report the synthesis and magnetic characterisation of a series of planar [M7] (M =
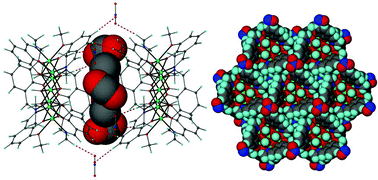
Abstract
The reactions of di-2-pyridyl ketone, (py)2CO, with [Cu2(O2CMe)4(H2O)2] in the presence of NaN3, HCl and HBr have led to the isolation of complexes {[Cu8{(py)2CO2}4(N3)6(O2CMe)2]·2MeCN·H2O}∞ (1·2MeCN·H2O), [Cu2{(py)2C(OH)O}Cl3]∞ (2) and [Cu2{(py)2C(OH)O}Br3]∞ (3), respectively, where (py)2 and (py)2C(OH)O− are the dianion and the monoanion of the gem-diol form of (py)2CO. Complex 1·2MeCN·H2O is an 1D coordination polymer consisting of centrosymmetric [Cu8{(py)2CO2}4(N3)6(O2CMe)2] cluster units linked through weakly coordinated azido bridges. The (py)2 groups adopt the η1:η2:η2:η1:μ4 coordination mode, while the ions behave as η2:μ and η1: η2:μ3 ligands. The isostructural compounds 2 and 3 are also 1D coordination polymers consisting of {Cu2{(py)2C(OH)O}X3}2 units (X=Cl, Br) linked through double halo bridges. The (py)2C(OH)O- ligand adopts the tridentate, bis-chelating η1:η2: η1:μ mode. A common feature in the three complexes is the presence of interchain H-bonding interactions which result in the formation of 2D networks. The magnetic properties of 1–3 have been studied by variable-temperature dc magnetic susceptibility and variable-field magnetization techniques. The analyses of the magnetic data were performed taking into account only the dominant exchange interactions within dinuclear subunits. The intradinuclear exchange interactions have been found to vary from strongly and moderately antiferromagnetic in 1 to ferromagnetic in 2 and 3. This work demonstrates the flexibility, versatility and synthetic potential of combining (py)2CO with carboxylate and azido or halo ligands.
The synthetic investigation of the general [Cu2(O2CMe)4(H2O)2]/(py)2CO/X− reaction system, where (py)2CO is di-2-pyridyl ketone and X− is Cl−, Br− or N3−, has led to 1D coordination polymers with interesting structures and magnetic properties.
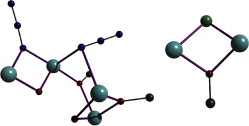
Abstract
We report three heptanuclear [
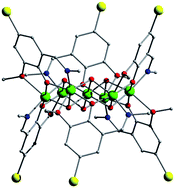
2009
Abstract
The synthesis and characterisation of a large family of trimetallic [MnIII3] Single-Molecule Magnets is presented. The complexes reported can be divided into three categories with general formulae (type 1) [MnIII3O(R-sao)3(X)(sol)3-4] (where R =
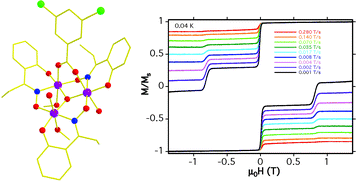
Abstract
The reactions of Ga(acac)3 with salicylaldoxime (saoH2) and methyl-salicylaldoxime (Me-saoH2) in dichloromethane/hexane afforded the complexes [Ga(acac)(saoH)2] (1) and [Ga(acac)3][Ga(acac)(MesaoH)2] (2), respectively, in high yields. The crystal structures of 1 and 2 have been determined by single-crystal X-ray crystallography. Both complexes are mononuclear with the Ga(III) atoms being in octahedral environments surrounded by two bidentate chelate R-saoH− and one bidentate chelate acac− ligands. A [Ga(acac)3] moiety has co-crystallized along with the methylsalicylaldoximato complex. Characteristic IR as well as NMR data are discussed in terms of the nature of bonding in the structures of the two complexes. 1H and 13C NMR data in CDCl3 indicate that the salicylaldoximato complexes isomerize in solution.
The reactions of Ga(acac)3 with salicylaldoxime (saoH2) and methylsalicyladoxime (MesaoH2) resulted in two mononuclear complexes with the general formula [Ga(acac)(RsaoH)2]. The structures of both complexes were determined with single-crystal X-ray crystallography. Both complexes were also characterized by IR, 1H and 13C NMR spectroscopy.
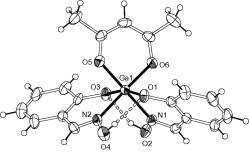
Abstract
The reactions of HgCl2 with 2,1,3-benzoselenadiazole (bsd) in methanol afforded the polymeric complexes [HgCl2(bsd)2]n (1) and [HgCl2bsd]n (2) in good yields. The crystal structures of 1 and 2 have been determined by single-crystal X-ray crystallography. Complex 1 is an one-dimensional (1D) polymer and consists of (HgCl2)n chains running parallel to the c-axes. Complex 2 is a two-dimensional (2D) polymer and consists of (HgCl2)n chains running parallel to the a-axes and being further bridged by bsd molecules to create a layer lying parallel to the ab plane. Both crystal structures are dominated by π···π interactions between the bsd molecules, while the presence of N···Se interactions increases the dimensionality in 1. Characteristic IR data are discussed in terms of the nature of bonding in the structures of the two complexes.
The employment of 2,1,3-benzoselenadiazole (bsd) in mercury(II) chemistry afforded the polymeric complexes [HgCl2(bsd)2]n (1) and [HgCl2bsd]n (2) Complex 1 is an one-dimensional (1D) coordination polymer, while 2 is a two-dimensional (2D) polymer. Both complexes consist of (HgCl2)n chains and are dominated by π···π interactions between the bsd molecules.
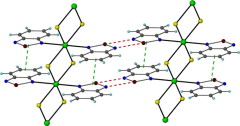
Abstract
The reactions of Ga(acac)3 with N-salicylidene-o-aminophenol (saphH2) and its 5-methyl (5MesaphH2) and 5-bromo (5BrsaphH2) derivatives in alcohols afforded the complexes [Ga(acac)(saph)(EtOH)] (1), [Ga(acac)(5Mesaph)(MeOH)] (2) and [Ga(acac)(5Brsaph)(EtOH)] (3), respectively, in good yields. The crystal structures of 1 and 2 have been solved by single-crystal X-ray crystallography. All three complexes are mononuclear with the GaIII atoms being surrounded by a dianionic tridentate Schiff base ligand, one bidentate acac− ligand and a terminal alcohol molecule. Characteristic IR data are discussed in terms of the nature of bonding and the structures of the three complexes.
Reactions of Ga(acac)3 with N-salicylidene-o-aminophenol (saphH2) and its 5-methyl and 5-bromo derivatives in alcohols (ROH) resulted in three mononuclear complexes with the general formula [Ga(acac)(5Rsaph)(ROH)]. The structures of the saph2− and 5Mesaph2− were determined with single-crystal X-ray crystallography while all complexes were characterized by IR spectroscopy and elemental analysis.
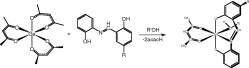
Abstract
The systematic investigation of the coordination chemistry of α-aminoisobutyric acid (H-Aib-OH) and Aib-based small peptides is continued. The solid complexes [Zn3(H-Aib-O)6]·MeCOOH·H2O (1·MeCOOH·H2O), {[Zn(H-Aib-L-Ala-O)2]·H2O}n (2·H2O) and [Zn(H2-Aib-Aib-Aib-O)4](ClO4)2·5.8H2O (3·5.8H2O) have been isolated and characterized by single-crystal X-ray crystallography. In the structure of complex 1·MeCOOH·H2O, three ZnII ions and six H-Aib-O− ligands have assembled to form a trinuclear cluster. All three ZnII centers are in a very distorted trigonal bipyramidal coordination. The trinuclear units assemble through a network of hydrogen bonds to form a 2D framework with a (3.6.3.6) topology, while the lattice acetic acid and water molecule connect the layers to create a 3D framework with a fcu topology. Complex 2·H2O is a two-dimensional coordination polymer. The deprotonated dipeptide behaves as a η1:η1:η1:μ2 ligand binding one ZnII atom through its amino nitrogen and peptide oxygen, and an adjacent ZnII atom through one of its carboxylate oxygen. In the crystal lattice, the layers are connected in the third direction through hydrogen bonds and the resulting framework conforms to a tfa net. Complex 3·5.8H2O consists of mononuclear [Zn(H2-Aib-Aib-Aib-O)4]2+ cations, and lattice water molecules. The tripeptide ligands are in their zwitterionic form and coordinate through one of the carboxylate oxygen atom to the metal ion, while they are participating in a network of intra- and intermolecular hydrogen bonds forming a 3D framework that adopts the bcu network.
The synthetic investigation of reaction systems containing ZnII and α-aminoisobutyric acid (H-Aib-OH) or Aib-based peptides gives products with interesting molecular and supramolecular structures.
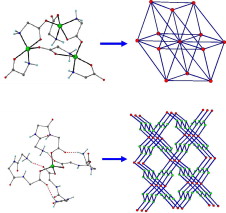
Abstract
The complexes [MnIII3O(Et-sao)3(O2CPh(Cl)2)(MeOH)3(H2O)] (1), [MnIII3O(Et-sao)3(ClO4)(MeOH)3] (2), [MnIII3O(Et-sao)3(O2Ph(CF3)2)(EtOH)(H2O)3] (3), and [MnIII3O(Ph-sao)3(O2C-anthra)(MeOH)4]·Ph-saoH2 (4·Ph-saoH2) display dominant ferromagnetic exchange interactions leading to molecules with S = 6 ground states. The molecules are single molecule magnets (SMM) displaying large effective energy barriers for magnetization reversal. In each case their crystal structures reveal multiple intermolecular H-bonding interactions. Single crystal hysteresis loop measurements demonstrate that these interactions are strong enough to cause a clear field bias, but too weak to transform the spin networks into classical antiferromagnets. These three-dimensional networks of exchange coupled SMMs demonstrate that quantum tunnelling magnetization can be controlled using exchange interactions, suggesting supramolecular chemistry can be exploited to modulate the quantum physics of molecular magnets.
Abstract
An attempt to utilize a Co(II) cubane cluster as a building block to create extended frameworks results instead in the transformation of the cube into a dimer of dimers.
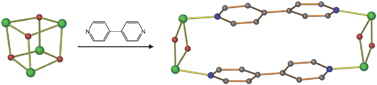
Polynuclear complexes based on paramagnetic metal ions offer the potential for new technological advances since many of them display fascinating physical properties.1–5 The species which are most promising for technological applications include single-molecule magnets,3 molecular coolants4 and spin phonon traps.5 The properties of such species are of molecular origin since it is the individual molecules that exhibit the physical properties rather than the bulk solids.1,2 Although it has been known for some time that intermolecular interactions, often weak, between molecules in the crystal lattice may affect the observed physical properties, few investigations of the importance and controllability of such interactions exist.2
We have recently initiated a project to study in detail the weak interactions between paramagnetic metal clusters and to utilize them as building blocks for the construction of extended frameworks.6 To this end, our attention turned toward the chemistry of di-2-pyridyl ketone [(py)2CO, Scheme 1] which, among others, has given rise to a number of tetranuclear clusters with a cubane topology where the metal atoms are arranged on the corners of a tetrahedron.7 In some of these clusters, ferromagnetic interactions between the metal ions are observed.8 Furthermore each metal centre has a potentially vacant coordination site, often occupied by a counter anion or a solvent molecule, which may be used as a connection site. We reasoned therefore that such tetranuclear clusters may serve as building blocks for the construction of extended frameworks, built on ferromagnetic clusters, if combined with linear bridging ligands such as 4,4′-bipyridine (4,4′-bpy, Scheme 1). Although many metal-organic frameworks are built on polynuclear metal complexes,9 there is only a handful of attempts where pre-isolated clusters have been utilized as building blocks for the construction of such materials.10 Tailoring of extended molecular materials still remains a challenge in this field. Aiming to build such an extended framework, we first synthesized the new Co(II) cubane cluster [Co4{(py)2C(OH)O}4(NO3)3(H2O)]NO3 (1), where (py)2C(OH)O− is the monoanion of the hydrate of (py)2CO, which we then further reacted with 4,4′-bpy. However, instead of obtaining an extended framework we isolated the tetranuclear assembly [Co4{(py)2C(OMe)O}4(4,4′bpy)2(MeOH)4](NO3)4·2MeOH (2·2MeOH), where (py)2C(OMe)O− is the monoanion of the hemiacetal of (py)2CO. The latter can be regarded as a dimer of dimers.11 Complexes 1 and 2 both show dominant ferromagnetic interactions between the Co(II) ions.
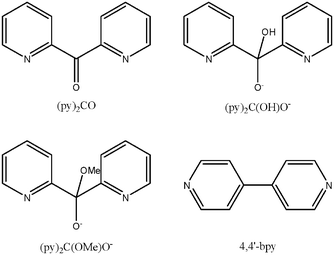
Abstract
The synthesis, single-crystal X-ray structure and magnetic properties of [Cu3L2Cl2(DMF)4]n (1), where L2− is the 2,3-dioxyquinoxalinate(-2) ligand, are reported. The complex was prepared by the reaction of CuCl2 and 1,4-dihydro-2,3-quinoxalinedione (H2L′) under basic conditions using either solvothermal or normal laboratory techniques. Compound 1 is a 3D coordination polymer with an (82.10)-a, lig (LiGe) topology, containing the ligand in a novel 3.1111 (Harris notation) coordination mode. Variable-temperature and variable-field magnetic studies reveal that the ligand L2− propagates weak antiferromagnetic exchange interactions through its “quinoxaline” part. IR data are discussed in terms of the structural features of 1 and the coordination mode of L2−.
The initial use of 1,4-dihydro-2,3-quinoxalinedione (H2L′) in CuII chemistry has led to a 3D coordination polymer featuring the 2,3-dioxyquinoxalinate (L2−) ligand; the complex has the extremely rare (82.10)-a, lig (LiGe) architecture and is characterized by the propagation of weak antiferromagnetic exchange interactions through the “quinoxaline” part of L2−.
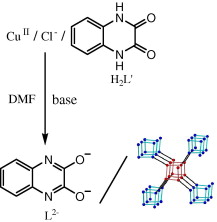
Abstract
The employment of the di-2-pyridyl ketone ligand combination in copper(II) chemistry has provided access to the hexanuclear cluster [Cu6(O2CPh)4(N3)2{(py)2CO2}2{(py)2C(OH)O}2] (1), and the coordination polymers [Cu4(O2CPh)2(N3)4{(py)2C(OMe)O}2]n (2) and [Cu2(O2CPh)(N3)2{(py)2C(OMe)O}]n (3). The structural type (cluster versus polymer) depends on the reaction solvent. Complex 1 has a [CuII6(μ3-OR′′)2(μ-OR′′)4]6+ core based on a central defective cubane unit and two additional CuII atoms across the missing edges. Complex 2 consists of zig-zag chains and complex 3 consists of extended 2D networks that adopt the herringbone architecture which is not based on T-shaped nodes. Cluster 1 is antiferromagnetically coupled with an S = 0 ground state.
The employment of the ternary ‘blend’ of ligands in copper(II) chemistry has provided access to a CuII6 cluster with a novel core and to two (CuII8)n and (CuII2)n coordination polymers (the former 1D, the latter 2D) depending on the reaction solvent; the products have unique structural characteristics.
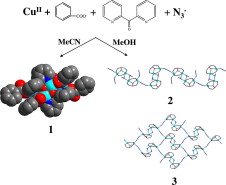
Abstract
The use of α–
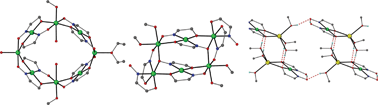
The synthesis and characterisation of polymetallic clusters of paramagnetic transition metal ions is an area of intense current research since such species may display fascinating physical phenomena. Indeed serendipitous self assembly has led to the discovery of, for example, single-molecule magnets, molecular coolants and spin phonon traps that have inspired much beautiful chemistry and physics with exciting potential application. Introducing new preparative routes towards the synthesis of transition metal clusters is thus of interest for the discovery of completely new structural types and as a means of building up families of related compounds so that structure–property relations can be developed. Organic ligands play a crucial role in successful cluster synthesis, but their nature and variety is surprisingly restricted. In the last few years we have been exploiting the coordination ability of salicyl-oximes (R-saoH2) to build Mn clusters, leading to the discovery of, amongst others, families of Mn3 and Mn6 molecules based on linked triangles. Recently we have turned our attention to the synthesis of Ni-oxime cages, and while R-saoH2ligands are excellent for M(III) cluster synthesis they are useless for building M(II) clusters since they form only mononuclear [M(R-saoH)2] type species—a fact exploited for some time in industrial metal extraction. Our first foray into Ni-oxime chemistry produced [Ni12] molecules using pyridine-2-amidoxime (IUPAC name: N-hydroxy-pyridine-2-carboxamidine) and here we extend this work to α-benzoin oxime (aboH2) and report the first members of a family of molecular and supramolecular wheels. Molecular wheels have long fascinated chemists not only for their architectural beauty but for their rich physical behaviour.Ni(II) wheels are extremely rare restricted to the species [Ni6], [Ni8], [Ni12] and [Ni24], while α-benzoin oxime has been sparingly employed in transition metal coordination chemistry—a search of the CCDC database revealing just one molybdenum dimer.
Abstract
N-salicylidene-o-aminophenol and two of its derivatives bearing either an electron-donating methyl group or an electron-withdrawing Br group were used as ligands for the synthesis of three Ga(III) complexes. The complexes involved the participation of one acetylacetonate and one ethanol or methanol molecule. The geometry of the dianion of the Schiff base in the complexes is planar while photoluminescence data showed that photoluminescence intensity was extensively increased upon complex formation. Complexes emitted ligand-centered luminescence by ligand-centered excitation. Substituent groups broadly modified emission maximum. These substitutions can be exploited to tune light emission by the complex. All three complexes were used for the construction of electroluminescence devices and all emitted electroluminescence. Both photoluminescence and electroluminescence emission was enhanced in the case of substituted Schiff bases.
Abstract
The chemistry of 1-hydroxybenzotriazole (btaOH) with the group 12 metal ions Zn(II) and Cd(II) resulted in the 1D coordination polymer [Zn(btaO)2]n (1) and the mononuclear complex [Cd(btaO)2(H2O)4] · 3H2O (2 · 3H2O), respectively. Polymer 1 forms double-stranded chains which are further supported by weak C–H⋯O hydrogen bonds resulting in a pcu (primitive cubic) rod packing. The mononuclear complexes of 2 form undulating hydrogen bonded layers while the lattice water molecules form 1D hydrogen bonded tapes, comprising 4- and 6-membered rings, within the layers and bridge them to the third dimension. The thermal and optical properties of both compounds are presented. IR data are discussed in terms of the chemical composition of the compounds and the coordination mode of btaO−.
The use of 1-hydroxybenzotriazole in the chemistry of group 12 metals afforded an 1D zinc(II) coordination polymer and a mononuclear cadmium(II) complex that forms undulating hydrogen bonded layers where lattice water molecules form 1D hydrogen bonded tapes, comprising 4- and 6-membered rings and bridge them to the third dimension.
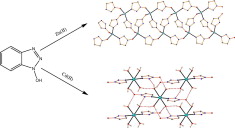
Abstract
The trinuclear nanomagnet [MnIII3O(Et-sao)3(MeOH)3](ClO4) (1) has been utilized as a building block for the construction of the hexanuclear cluster [{MnIII3O(Et-sao)3(O2CPh)(EtOH)}2{4,4′-bpe}2] (3) that conforms to a rectangle and the two-dimensional coordination polymer {[MnIII3O(sao)3(4,4′-bpe)1.5]ClO4·3MeOH}n (2·3MeOH). The latter exhibits an unprecedented type of entanglement that is based on host guest interactions. The polygon versus the polymer is rationalized in terms of changing an auxiliary anion that influences the arrangement of the potentially “vacant” coordination axes on each MnIII ion of the trinuclear precursor and thereby directing the self-assembly process.
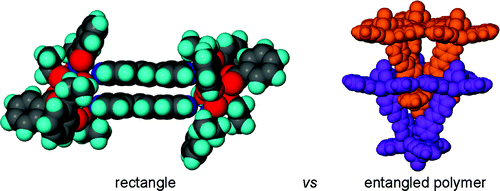
2008
Abstract
Metal−organic polyhedra with surface-exposed organic groups have been designed. The polyhedra are based on concentric shells of alternating negative−positive−negative charges and have been used to design homochiral hosts.

Abstract
The reactions of di-2-pyridyl ketone with Cu(O2CPh)2 in the presence of NaN3 and LiOH have led to an antiferromagnetically coupled (S = 0) CuII6 cluster with a novel core and to (CuII8)n and (CuII2)n coordination polymers (the former 1D and the latter 2D) with interesting structures. The cluster or polymer formation depends on the reaction solvent.
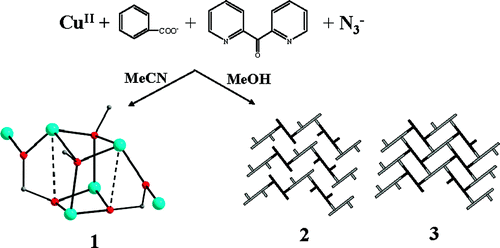
Abstract
We report the synthesis of a series of mixed valence MnII/IV tetranuclear clusters [MnII2MnIV2O2(heed)2(EtOH)6Br2]Br2 (1), [MnII2MnIV2O2(heed)2(H2O)2Cl4]·2EtOH·H2O (2·2EtOH·H2O), [MnII2MnIV2O2(heed)2(heedH2)2](ClO4)4 (3), [MnII2MnIV2O2(heed)2(MeCN)2(H2O)2(bpy)2](ClO4)4 (4) and [MnII2MnIV2O2(heed)2(bpy)2Br4]·2MeOH (5·2MeOH). Clusters 1–5 are constructed from the tripodal
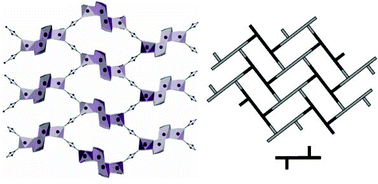
Abstract
Two new organotin(IV) complexes with dianionic dipeptides containing the α-aminoisobutyryl residue (Aib) as ligands are described. The solid complexes [(n-Bu)2Sn(H−1LA)] · 2MeOH (1 · 2MeOH) (LAH = H-Aib-L-Leu-OH) and [(n-Bu)2Sn(H−1LB)] · MeOH (2 · MeOH) (LBH = H-Aib-L-Ala-OH) have been isolated and characterized by single-crystal X-ray crystallography and spectroscopic techniques (H−1L2− is the dianionic form of the corresponding dipeptide). Complexes 1 · 2MeOH and 2 · MeOH are monomeric with similar molecular structures. The doubly deprotonated dipeptide behaves as a N(amino), N(peptide), O(carboxylate) ligand and binds to the SnIV atom. The five-coordinate metal ion has a distorted trigonal bipyramidal geometry. A different network of intermolecular hydrogen bonds in each compound results in very dissimilar supramolecular features. The IR, far-IR, Raman and 119Sn NMR data are discussed in terms of the nature of bonding and known structures. The antibacterial and antiproliferative activities as well as the effect of the new compounds on pDNA were examined. Complexes 1 and 2 are active against the gram-positive bacteria Bacillus subtilis and Bacillus cereus. The IC50 values reveal that the two compounds express promising cytotoxic activity in vitro against a series of cell lines.
Abstract
We describe how reactivity can be controlled in the solid state using molecules and self-assembled metal−organic complexes as templates. Being able to control reactivity in the solid state bears relevance to synthetic chemistry and materials science. The former offers a promise to synthesize molecules that may be impossible to realize from the liquid phase while also taking advantage of the benefits of conducting highly stereocontrolled reactions in a solvent-free environment (i.e., green chemistry). The latter provides an opportunity to modify bulk physical properties of solids (e.g., optical properties) through changes to molecular structure that result from a solid-state reaction. Reactions in the solid state have been difficult to control owing to frustrating effects of molecular close packing. The high degree of order provided by the solid state also means that the templates can be developed to determine how principles of supramolecular chemistry can be generally employed to form covalent bonds. The paradigm of synthetic chemistry employed by Nature is based on integrating noncovalent and covalent bonds. The templates assemble olefins via either hydrogen bond or coordination-driven self-assembly for intermolecular [2 + 2] photodimerizations. The olefins are assembled within discrete, or finite, self-assembled complexes, which effectively decouples chemical reactivity from effects of crystal packing. The control of the solid-state assembly process affords the supramolecular construction of targets in the form of cyclophanes and ladderanes. The targets form stereospecifically, in quantitative yield, and in gram amounts. Both [3]- and [5]-ladderanes have been synthesized. The ladderanes are comparable to natural ladderane lipids, which are a new and exciting class of natural products recently discovered in anaerobic marine bacteria. The organic templates function as either hydrogen bond donors or hydrogen bond acceptors. The donors and acceptors generate cyclobutanes lined with pyridyl and carboxylic acid groups, respectively. The metal−organic templates are based on Zn(II) and Ag(I) ions. The reactivity involving Zn(II) ions is shown to affect optical properties in the form of solid-state fluorescence. The solids based on both the organic and metal−organic templates undergo rare single-crystal-to-single-crystal reactions. We also demonstrate how the cyclobutanes obtained from this method can be applied as novel polytopic ligands of metallosupramolecular assemblies (e.g., self-assembled capsules) and materials (e.g., metal−organic frameworks). Sonochemistry is also used to generate nanostructured single crystals of the multicomponent solids or cocrystals based on the organic templates. Collectively, our observations suggest that the organic solid state can be integrated into more mainstream settings of synthetic organic chemistry and be developed to construct functional crystalline solids.
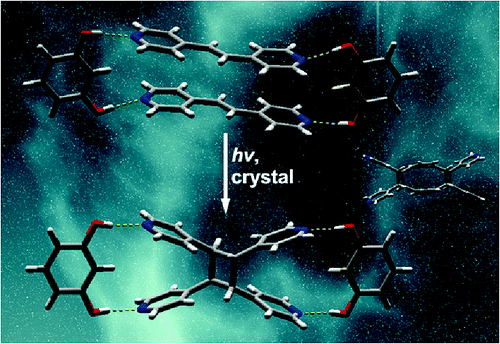
Abstract
Tetratopic rctt-tetrakis(4-pyridyl)cyclobutane acts as an angular bifunctional
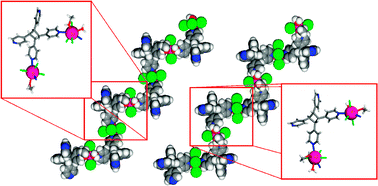
2007
Abstract
The 12: 1 reaction of urea (U) with CoI2 in EtOH yielded the “clathrate-coordination” compound
Abstract
In this microreview, we show how principles of coordination‐driven self‐assembly can be used to direct chemical reactivity in the organic solid state. We also show how products obtained from the templated solid‐state reactions can be used as organic building units of metal–organic frameworks and coordination capsules. Self‐assembled structures based on dinuclear ZnII and AgI complexes are used to direct stereocontrolled [2+2] photodimerizations of vinylpyridines in the solid state in up to quantitative yield. The products are used as bi‐ and polytopic ligands of self‐assembled coordination capsules and metal–organic frameworks. We also describe emerging metal‐based approaches to conduct mechanochemical reactions in organic solids. In addition to the field of supramolecular coordination chemistry, these observations impact the field of green chemistry.(© Wiley‐VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, Germany, 2007)
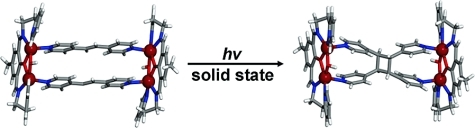
Abstract
Addition of rctt-tetrakis(2-pyridyl)cyclobutane (2,2‘-tpcb) in a CuII/N3- solution afforded the 1D coordination polymer [Cu3(N3)6(2,2‘-tpcb)(DMF)2]n (1). The ligand 2,2‘-tpcb serves as a tetradentate bis-chelating ligand by linking linear [(DMF)Cu(μ1,1-N3)2Cu(N3)2(μ1,1-N3)2Cu(DMF)] trinuclear units to produce a zigzag chain. Within each centrosymmetric trinuclear unit there exist two irregularly asymmetric end-on double azido-bridged [Cu(μ1,1-N3)2Cu]2+ cores, while one of the largest Cu−Nazide−Cu angles is observed. Magnetic susceptibility data, measured from 2 to 300 K, show bulk moderate ferromagnetic coupling within the magnetically isolated trinuclear units. These data were fitted to the appropriate equation derived from the Hamiltonian H = −J1(SA1SB + SA2SB) − J2SA1SA2, giving the parameters J1 = +70(3) cm-1, J2 = −3(2) cm-1, g = 2.12(1), with an intertrimer interaction parameter θ = −0.74(2) K. The coupling constants were correlated with the structural parameters. Density functional calculations reproduce very well the experimental J values and show that ferromagnetism for this complex is mainly due to the topology of the magnetic orbitals and the different coordination spheres of two neighboring CuII atoms, resulting in a small overlap of the orbitals possessing the unpaired electrons.
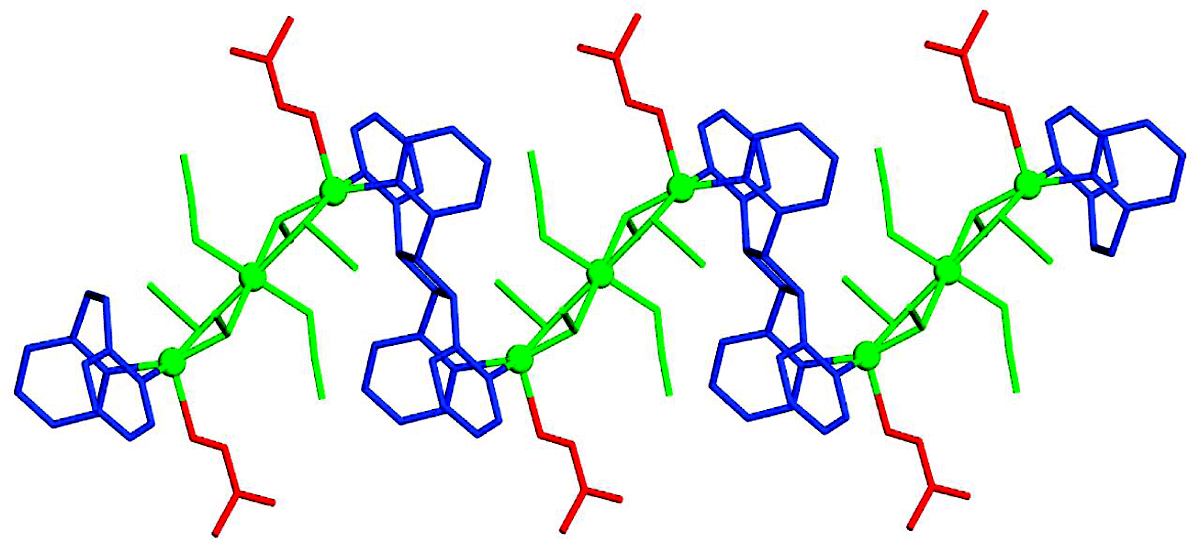
Abstract
Reactions of
Abstract
The reactions of Ga(NO3)3 · 9H2O with 2,2′-bipyridine (bpy), 1,10-phenanthroline (phen) and 2,2′:6′,2″-terpyridine (terpy) in alcohols afford the complexes [Ga2(OH)2(bpy)4](NO3)4 (1), [Ga2(OH)2(phen)4](NO3)4 (2) and [Ga2(OH)2(H2O)2(terpy)2](NO3)4 (3), respectively, in good yields. The crystal structures of 1 · 1.3MeOH · 1.4H2O, 2 · 4.5MeOH and 3 have been solved by single-crystal X-ray crystallography. The three complexes contain the core. Four nitrogen atoms from two chelating bpy (1) or phen (2) complete distorted octahedral coordination at each metal centre. In 3 one tridentate chelating terpy and one aquo ligand complete six coordination at each GaIII atom. The crystal structure of 2 · 4.5MeOH is stabilized by hydrogen bonds and intermolecular π–π stacking interactions, while in 3 hydrogen bonds create a double-chain, ladder-like architecture. Characteristic IR data are discussed in terms of the nature of bonding and the structures of the three complexes. The 1H NMR spectra of complexes 2 and 3 suggest the presence of the free ligand and one solution species containing the coordinated ligand. The structure of the cation [Ca2(OH)2(bpy)4]4+ is retained in D2O as deduced by the 2D homonuclear COSY map.
Abstract
A general method of increasing the ground-state total spin value of a polynuclear 3d-metal complex is illustrated through selected examples from cobalt(II) and nickel(II) cluster chemistry that involves the dianion of the gem-diol form of di-2-pyridyl ketone and carboxylate ions as organic ligands. The approach is based on the replacement of hydroxo bridges, that most often propagate antiferromagnetic exchange interactions, by the end-on azido ligand, which is a ferromagnetic coupler.
2006
Abstract
Treatment of a methanolic solution of gallium(III) nitrate with lithium hydroxide in the presence of benzilic acid resulted in the decanuclear cluster [Ga(OMe)2{O2CC(OH)Ph2}]10 (1). The metal and the organic components have assembled to form a cyclic molecule that adopts the structure of a wheel. The 10 GaIII ions are approximately coplanar and are coordinated in a distorted octahedral manner by six oxygen atoms. The integrity of the molecular wheel is retained in solution, as evidenced by the 1NMR spectrum of 1 in DMSO-d6, while no signal in the 71Ga NMR could be detected.
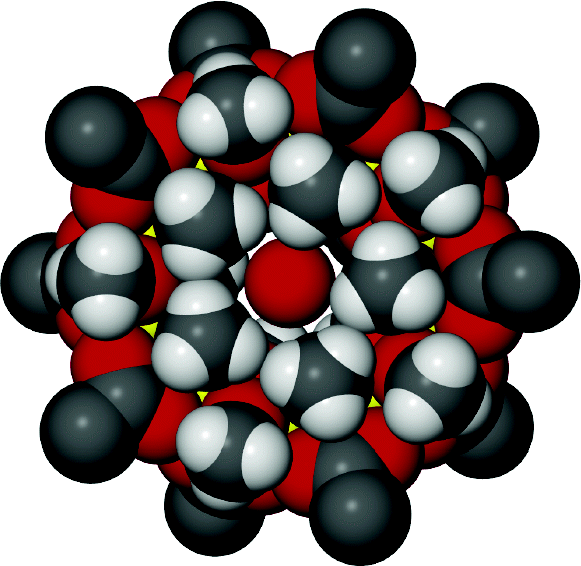
Abstract
We show that a molecule constructed from an organic solid-state synthesis, namely, rctt-1,3-bis(2-pyridyl)-2,4-bis(4-pyridyl)cyclobutane (ht-2,4′-tpcb) upon reaction with Cu(II) and Zn(II) ions gives one- (1D) and two-dimensional (2D) metal–organic frameworks (MOFs) of composition [Cu(hfac)2(2,4′-tpcb-ht)]∞ (where: hfac=hexafluoroacetylacetonate) (1) and [Zn(2,4'-tpcb-ht)2(H2O)]2+∞ (2). Each MOF possesses uncoordinated, or free, 2-pyridyl groups that decorate the walls of each extended structure.
2005
Abstract
Transition-metal-ion complexes involving seven-membered chelation rings have emerged as important for applications in catalysis and biology. Specifically, N-C4-N bridged metallocycles [1] have found applications as catalysts [2] and models of biological compounds [3], as well as antibacterial [4] and antitumor [5] agents. In terms of catalysis, the application of chelating N-donor ligands as compared to analogous polyphosphines has begun to attract attention, especially for the activation of C-H bonds [6]. Thus, ligands that provide more than one pyridyl group in close proximity are being studied as preorganized metal chelation units [6b]. In addition, there is increasing interest in the development of novel non-metallocene catalysts for olefin polymerization for which complexes involving N-C4-N bridged chelate rings have been effective [2c]. Therefore, studies that involve N-C4-N ligands with a potential to offer seven-membered chelation are of much current interest.
Abstract
An undergraduate laboratory experiment that employs molecules, in the form of linear hydrogen-bond templates, to direct the [2 + 2] photodimerization in the solid state is described. The experiment introduces the undergraduate students to supramolecular and solid-state chemistry, as well as aspects of green chemistry.
Abstract
A metal organic framework with two different nodes (circle and square) and a structure related to one of the 20 known 2-uniform nets has been constructed using an organic building unit that codes for multiply fused nodes.
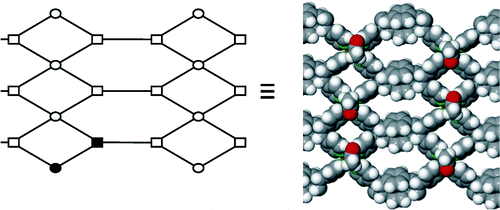
Abstract
A ladder-like coordination polymer involving a dinuclear Zn Schiff-base complex possesses organic linkers that undergo [2 + 2] photodimerisation in the solid state.
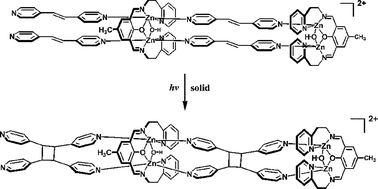
Abstract
The use of di‐2‐pyridyl ketone [(2‐py)2CO]/dicyanamide “blend” in cobalt(II), manganese(II) and nickel acetatechemistry has yielded neutral cubane clusters. The preparation of [Mn4(O2CMe)2{N(CN)2}2{(2‐py)2C(OH)O}4]·2(2‐py)2‐CO·4H2O (1) and [M4(O2CMe)2{N(CN)2}2{(2‐py)2C(OH)O}4]· 10H2O [M = Ni (2), Co (3)] was achieved by the reaction of [M(O2CMe)2]·4H2O with (2‐py)2CO and Na[N(CN)2] in MeOH/H2O (1:5, v/v) at room temperature. The metal(II )‐mediated hydrolysis of (2‐py)2CO to give the coordinated monoanion of the hydrate gem ‐diol form involves a nucleophilic attack of H2O on the carbonyl group. In the case of the cobalt reaction system, there is a second product. Upon employing an excess of (2‐py)2CO, long reaction times, reflux conditions and high dilution, slow oxidation of CoII takes place leading to the isolation of the mononuclear cobalt(III ) complex [Co{(2‐py)2C(OH)O}2][N(CN)2]· 4H2O (4) in yields higher than 70 %. The structures of 1, 2 and 4 have been solved by single‐crystal X‐ray diffraction studies, while a unit‐cell determination indicated that complex 3 is isostructural with 2. The tetranuclear cluster molecules of 1 and 2 have cubane [M4(μ3‐OR)4]4+ cores with divalent metal atoms and deprotonated oxygen atoms [originating from the(2‐py)2C(OH)O– ligands] occupying alternate vertices. The centrosymmetric [Co{(2‐py)2C(OH)O}2]+ cation of complex 4 has an octahedral structure with the (2‐py)2C(OH)O– ligands adopting the tridentate N ,O ,N ‐chelating coordination mode. The magnetic properties of 1–3 have been studied by variable‐temperature dc magnetic susceptibility techniques. The studies indicate weak antiferromagnetic coupling for 1 and ferromagnetic behaviour for 2 and 3. A three‐J model was found to be adequate for describing the thermal variation of the molar magnetic susceptibilities of 1 and 2. The magnetochemical results have been compared with literature data. (© Wiley‐VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, Germany, 2005)
.jpg)
Abstract
We show how a template-controlled reaction performed in the organic solid state can be used to construct a molecule that functions as an organic building unit of both a metal-organic polyhedron and polygon. The template is a small organic molecule that organizes two olefins via hydrogen bonds for a [2+2] photodimerization. The process of utilizing a molecule to build a molecule that is subsequently used for self-assembly is inspired by the general two-step process of template-directed synthesis and self-assembly of Nature that is used to construct large, functional self-assembled structures.
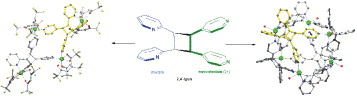
2004
Abstract
Catechol (cat) and trans-1,2-bis(4-pyridyl)ethylene (4,4’-bpe) form a 1:1 co-crystal, of composition (cat)·(4,4’-bpe) 1, that consists of infinite one-dimensional (1D) undulating chains held together by O-H···N hydrogen bonds. The chains are based on an alternating sequence of five crystallographically independent molecules, two that involve cat and three that involve 4,4’-bpe, and an eight-component repeat unit. The cat molecules adopt an anti-anti conformation, while the olefins adopt either a co-planar or twisted conformation. The chains self-assemble via a combination of face-to-face π-π interactions, C-H···π forces, and C-H···O hydrogen bonds.
Abstract
As part of our interest into the bioinorganic chemistry of gallium, gallium(III) complexes of the peptide ligand N-(2-(4-imidazolyl)ethyl)pyridine-2-carboxamide (pypepH2) resembling a fragment of the metal-binding domain of bleomycins (BLMs), have been isolated. Reaction of pypepH2 with (Et4N)[GaCl4] and Ga(acac)3 [acac− is the acetylacetonate(−1) ion] affords the mononuclear complex [Ga(pypepH)2]Cl · 2H2O (1) and the tetranuclear complex [Ga4(acac)4(pypep)4] · 4.4H2O (2), respectively. Both complexes were characterized by single-crystal X-ray crystallography, IR spectroscopy and thermal decomposition data. The pypepH− ion in 1 behaves as a N(pyridyl), N(deprotonated amide), N(pyridine-type imidazole) chelating ligand. The doubly deprotonated pypep2− ion in 2 behaves as a N(pyridyl), N(deprotonated amide), N(imidazolate), N′(imidazolate) μ2 ligand and binds to one GaIII atom at its pyridyl, amide and one of the imidazolate nitrogens, and to a second metal ion at the other imidazolate nitrogen; a chelating acac− ligand completes six coordination at each GaIII centre. The IR data are discussed in terms of the nature of bonding and known structures. The 1H NMR spectrum of 1 suggests that the cation of the complex maintains its integrity in dimethylsulfoxide (DMSO) solution. Complexes 1 and 2 are the first synthetic analogues of metallobleomycins with gallium(III).
Abstract
The application of molecular templates to direct reactivity in the solid state is described. Specifically, molecules that function as linear templates are demonstrated to provide control of the [2+2] photodimerization in the solid state such that it is possible to use the templates to conduct target-oriented molecular syntheses, or molecular syntheses by design. The degree of organization provided by the solid state is also shown to provide chemists a reliable means to capture and transform ordered complexes formed by way of hydrogen-bonded-driven self-assembly into complex covalent molecular and polymeric structures.
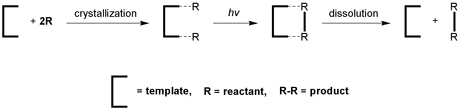
Abstract
Coordination-driven self-assembly is employed to direct a single-crystal-to-single-crystal [2 + 2] photodimerization that exhibits tunable fluorescence.
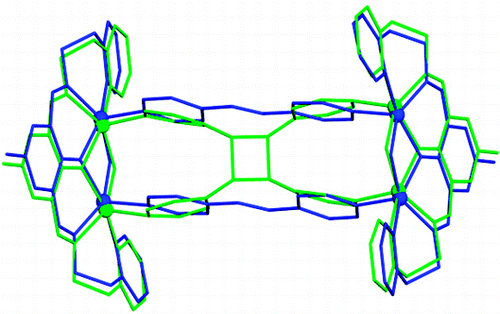
Abstract
The reaction of Ga2(SO4)3·18H2O and excess 2,2′:6′,2″-terpyridine (terpy) in MeOH / H2O leads to [Ga(OH)(SO4)(terpy)(H2O)]·H2O (1·H2O] in good yield. The structure of the complex has been determined by single-crystal X-ray crystallography. The GaIII atom in 1·H2O is 6-coordinate and ligation is provided by one terdentate terpy molecule, one monodentate sulfate, one terminal hydroxide and one terminal H2O molecule; the coodination polyhedron about the metal is described as a distorted octahedron. There is an extensive hydrogen-bonding network in the crystal structure which generates corrugated layers parallel to bc. The new complex was characterized by IR and 1H NMR spectroscopy. The spectroscopic data are discussed in terms of the nature of bonding
Abstract
The crystal and molecular structure of Rebek's imide 1 is reported. Crystal data for 1: triclinic, space group , a = 7.8733(7) Å, b = 12.712(1) Å, c = 12.789(1) Å, α = 86.628(5)°, β = 84.628(5)°, γ = 72.981(5)°, V = 1217.9(2) Å3, and Dc = 1.305 g/cm3 for Z = 2 and R = 0.047. The molecule crystallizes as a cyclic, hydrogen-bonded dimer held together by two NH...O and two OH...O hydrogen bonds involving amide...carboxylic acid interactions. The dimer organizes in the solid state to form 1D ribbons.
Abstract
The synthesis and crystal structure of the metal-organic framework {[Co(acetate)2(rctt-tetrakis(4-pyridyl)cyclobutane)] · 2(methanol)}∞ (1) are described. The structure of 1 is based on a (4,4)-grid topology with Co(II) ions and 4,4′-tpcb cyclobutane molecules as 4-connected nodes and two different rhombus-shaped cavities in the framework. Solvent methanol molecules are clathrated between the grids. A model involving pseudo-merohedral twinning was required to arrive at a satisfactory single-crystal structure solution. Crystal data for 1: triclinic, space group P, a=9.006(2), b=9.127(2), c=9.129(2), α=78.57(3), β=88.99(3), γ=88.98(3), V=735.4(3), and Z=1 for R=0.0362 (2539 reflections with I>2σ(I), 184 parameters).
Abstract
Di- 1a and tetranuclear 1b metal–organic squares have been obtained from bis- and tetra(pyridyl)cyclobutanes synthesized in the solid state, respectively; for 1b, a cyclobutane with two different metal binding subunits supports the formation of a polygon and polyhedron.
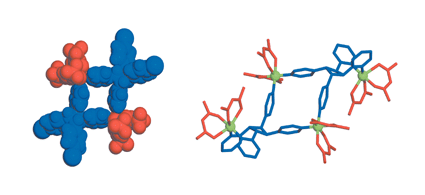
Abstract
Reaction of Ga(acac)3 with 5-hydroxyorotic acid dihydrate (5-hydroxy-2,6-dioxo-1,2,3,6-tetrahydropyridimine-4-carboxylic acid dihydrate, LH4 · 2H2O) in DMF yields the mononuclear complex (CH3)2NH2[Ga(LH2)2(DMF)2] · H2O · 0.4DMF (1). X-ray crystallographic analysis showed that the octahedral anion [Ga(LH2)2(DMF)2]− self-assembles through hydrogen bonds to form a 2D anionic framework; the cation (CH3)2NH2+ derives from the hydrolysis of the solvent. Complex 1 is the first crystallographically characterized 5-hydroxyorotate complex of any metal, and also the first Ga(III) complex with an orotate-type ligand.
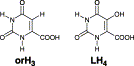
2003
Abstract
Metal–organic frameworks (MOFs) are crystalline inorganic–organic hybrid materials that consist of metal ions and organic molecules connected in space to produce an infinite one-, two-, or three-dimensional framework. The modularity of MOFs, specifically, the ability to modify the organic and/or inorganic components, offers a ready means to modify and control properties of such materials (e.g. inclusion, magnetism). This review highlights the design and synthesis of cavity-containing and porous MOFs with emphasis on methods that enable the functionalization of interior void spaces with organic groups. A relatively new class of MOFs, known as inverted metal–organic frameworks (IMOFs), which enables organic functionalization using principles of supramolecular chemistry, is discussed. We also briefly outline methods to functionalize the interior spaces of mesoporous materials (MCMs) and zeolites, and suggest that MOFs offer a diverse space within which to place a wide range of organic functionalities.
Abstract
A project related to the crystal engineering of hydrogen-bonded coordination complexes has been initiatied and some of our first results are presented here. The compounds [Mn(DMU)6](ClO4)2 (1), [Ni(DMU)6](ClO4)2 (2), [Cu(OClO3)2(DMU)4] (3) and [Zn(DMU)6](ClO4)2 (4) have all been prepared from the reaction of N,N′-dimethylurea (DMU) and the appropriate hydrated metal perchlorate salt. Crystal structure determinations of the four compounds demonstrate the existence of [M(DMU)6]2+ cations and ClO4 − counterions in (1), (2) and (4), whereas in (3) monodentate coordination of the perchlorate groups leads to molecules. The [M(DMU)6]2+ cations and ClO4 − anions self-assemble to form a hydrogen-bonded one-dimensional (1D) architecture in (1) and different 2D hydrogen-bonded networks in (2) and (4). The hydrogen bonding functionalities on the molecules of (3) create a 2D structure. The complexes were also characterised by room-temperature effective magnetic moments and i.r. studies. The data are discussed in terms of the nature of bonding and the known structures.
Abstract
In order to examine the possibilities of using lanthanide(III) ions in the crystal engineering of hydrogen bonded coordination complexes, the compounds [Ln(DMU)6][LnCl6] and [Ln(NO3)3(DMU)3] (Ln=Pr, Nd, Gd, Er; DMU=N,N′-dimethylurea) have been prepared from the reactions of DMU with the appropriate lanthanide(III) salts in alcohols. The representative complexes [Nd(DMU)6] [NdCl6] (2) and [Nd(NO3)3(DMU)3] (6) have been structurally characterised by single-crystal X-ray studies. The structure of 2 consists of distorted octahedral [Nd(DMU)6]3+ and [NdCl6]3− ions. In the molecules of 6, the Nd(III) ion is in a nine-coordinate, monocapped square antiprismatic geometry, surrounded by three O-bonded DMU ligands and three bidentate chelating nitrate groups. The [Nd(DMU)6]3+ cations and [NdCl6]3− anions self-assemble to form a hydrogen-bonded 3D architecture in 2. The hydrogen bonding functionalities on the molecules of 6 create also a 3D structure. Two main motifs of interionic/intermolecular hydrogen bonds have been observed: NH⋯Cl in 2 and NH⋯O(NO3 −) in 6; weak CH⋯Cl hydrogen bonding interactions are also present in 2. The complexes were characterised by magnetic susceptibilities at room temperature and spectroscopic (IR, far-IR, Raman) techniques. The vibrational data are discussed in terms of the nature of bonding and the known structures of the neodymium(III) complexes.
In the crystal structure of [Nd(DMU)6][NdCl6], the hexakis(N,N′-dimethylurea)neodymium(III) cation acts as a hydrogen-bonding building block with multi-fold connectivity linking [NdCl6]3− to generate 3D architectures. The hydrogen bonding functionalities on the molecules of [Nd(NO3)3(DMU)3] yield also 3D architectures without the intervention of anionic intermediares.
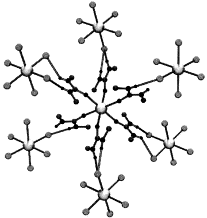
Abstract
The ligand N.N'-dimethylurea (DMU) is used to propagate the octahedral coordination geom- etry of [Co(DMU)6]2+ into 1D and 2D assemblies via a combination of coordinative bonds and interionic hydrogen-bonding. Compounds [Co(DMU)6](ClO4)2 (1), [Co(DMU)6](BF4)2 (2) and [Co(DMU)6](NO3)2 (3) have been prepared from the reactions of DMU and the appropriate hydrated cobalt(II) salts in EtOH. MeCN or Me2CO (only for 1) in the presence of 2,2-di- methoxypropane. Crystal structure determinations demonstrate the existence of [Co(DMU)6]2+ cations and CIO4- , BF4- or NO3- counterions. The great stability of the [Co(DMU)6]2+ cation in the solid state is attributed to a pseudochelate effect which arises from the existence of strong intracationic N-H···O(DMU) hydrogen bonds. The [Co(DMU)6]2+ cations and counterions self- assemble to form a hydrogen-bonded ID architecture in 1, and different 2D hydrogen-bonded networks in 2 and 3. The precise nature of the resulting supramolecular structure is influenced by the nature of the counterion. Two main motifs of intermolecular (interionic) hydrogen bonds have been observed: N-H ···O(ClO4-, NO3-) or N-H ··· F(BF4-) and weak C-H F(BF4- ) or C-H-O(NO3- ) hydrogen bonds. The complexes were also characterized by vibrational spec- troscopy (IR, far-IR. low-frequency Raman). The spectroscopic data are discussed in terms of the nature of bonding and the know;n structures.
2002
Abstract
Co-crystallization of 1,2-dihydroxybenzene (cat) with trans-1-(2-pyridyl)-2-(4-pyridyl)ethylene (2,4-bpe) gives a layered ternary solid, (cat)·(2,4-bpe)·(H2O) 1, with molecular components held together by a combination of O–H⋯N and O–H⋯O forces wherein 2,4-bpe is organized for a ‘head-to-tail’ (ht) [2+2] photocyclodimerization. UV-irradiation of the solid produces rctt-1,3-bis(2-pyridyl)-2,4-bis(4-pyridyl)cyclobutane [rctt-(2,4-tpcb-ht)] in quantitative yield. The structure of rctt-(2,4-tpcb-ht), in the form of crystalline rctt-(2,4-tpcb-ht)·1.5(H2O), has been confirmed by way of single-crystal X-ray diffraction.
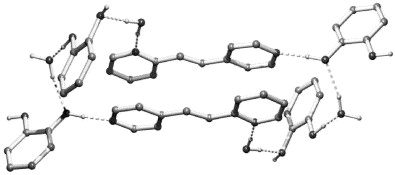
Abstract
A project related to the crystal engineering of hydrogen bonded coordination complexes has been initiated and our first results are presented here. The cis and trans forms of monothiomalonamide (LH2) have been fully characterized by vibrational spectroscopy, thermal techniques and single-crystal X-ray diffraction. The cis form crystallizes in the monoclinic space group P21/c and the trans form in the monoclinic space group C2/c. The respective lattice constants are a = 5.602(3), b = 9.055(2), c = 10.945(5)Å , β = 101.29(2)" (cis-LH2) and a = 20.336(7), b = 4.317(1), c = 12.432(5) Å, β = 92.16(1)" (trans-LH2). The new complexes [Pd(LH2)4]X2 (X = Cl, Br), [Pt(LH2)4]X2 (X = Br, I), [PdX2(LH2)2] (X = Cl, Br, I) and [PtX2(LH2)2] (X = Cl, I) were prepared and characterized by elemental analyses and spectroscopic (IR, far-IR, Raman) studies. All data are discussed in terms of the nature of bonding in conjunction with assigned structures. The LH2 molecule behaves as a monodentate ligand binding through the sulfur atom. Monomeric square planar structures are assigned for the metal complexes in the solid state; the 1:2 complexes have the trans geometry. A detailed comparison of the new complexes with the corresponding monothiooxamide complexes is also presented.
Abstract
The reaction of Co(O2CPh)2 with di-2-pyridyl ketone, (py)2CO, and NaN3 in DMF allows isolation of the tetranuclear compound [Co4(N3)2(O2CPh)2{(py)2C(OH)O}4]·2DMF (2) instead of the expected nonanuclear cage [Co9(N3)2(O2CPh)8{(py)2CO2}4]. The X-ray diffraction analysis reveals a defective double-cubane, tetrameric entity in which the CoII atoms are linked by η1,μ2-N3 azide ligand and two kinds of O-bridges. The molar magnetic susceptibility measurements of 2 in the 2–300 K range indicate bulk ferromagnetic coupling. Some synthetic aspects of the CoII/(py)2CO/N3 − reaction system are also discussed.
The tetranuclear compound [Co4(N3)2(O2CPh)2{(py)2C(OH)O}4]·2DMF (2) has been prepared by the reaction of Co(O2CPh)2 with di-2-pyridyl ketone, (py)2CO, and NaN3 in DMF. The X-ray diffraction analysis reveals a defective double-cubane entity in which the CoII atoms are linked by η1,μ2-N3 azide ligands and two kinds of O-bridges. The molar magnetic susceptibility measurements indicate bulk ferromagnetic coupling. Some synthetic aspects of the CoII/(py)2CO/N3 − reaction system are discussed.
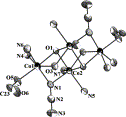
Abstract
A linear template is used to direct the synthesis of a “head-to-head” tetrapyridine in the solid state, which, in a second step, assembles with a transition-metal-ion to form a polyhedral metal-organic host with a structure that conforms to a trigonal antiprism. The application of the linear template is reminiscent of the synthetic strategy of nature.
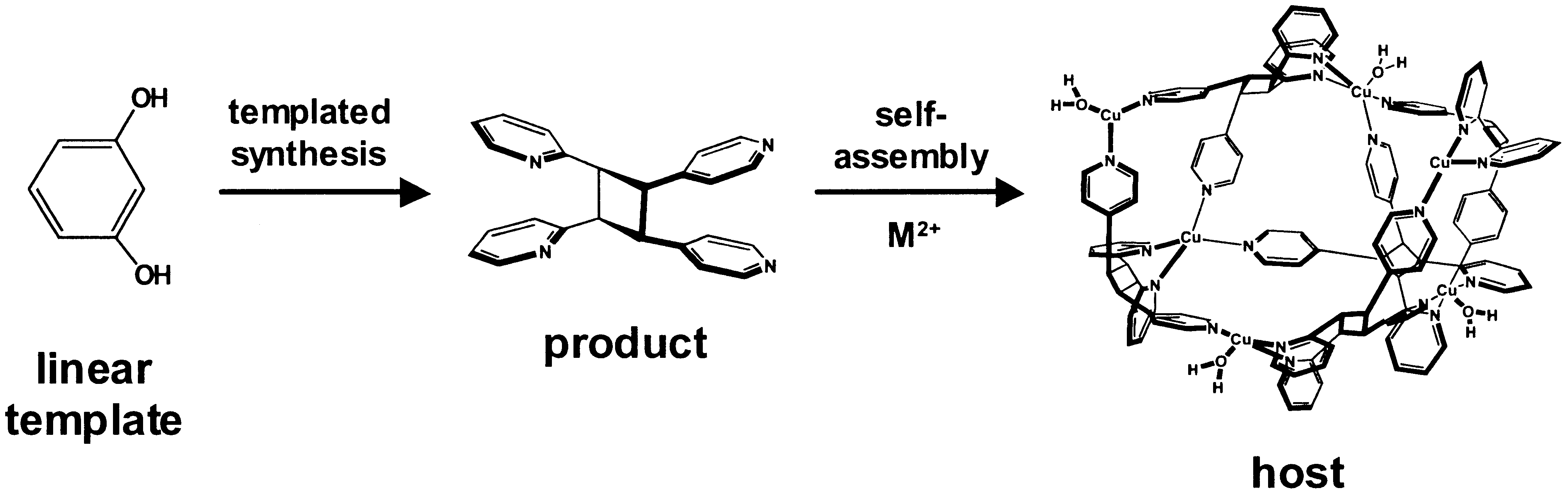
Abstract
The crystal and molecular structure of the title compound, [Cu2(3,5-dihydroxybenzoate)4 (acetonitrile)2] ⋅ 8H2O 1, is reported. Crystal data for 1: tetragonal, space group I 4/m, a = 11.720(2) Å, c = 15.304(3) Å, V = 2102.4(6), and Dc = 1.53 g/cm3, for Z = 2. The metal and organic components crystallize to form a Cu paddle-wheel complex, of idealized D4h symmetry, that assembles in the solid-state, along with eight equivalents of water, to form a 3D hydrogen-bonded network held together by O–H ⋅⋅⋅ O hydrogens bonds. The metal complexes pack to form a 2D layered structure.
Abstract
A product derived from a template-directed, organic, solid-state synthesis, rctt-tetrakis(2-pyridyl)cyclobutane (2,2′-tpcb), serves as a bis-chelating
Abstract
Abstract
The reaction of zerovalent manganese with 1‐hydroxybenzotriazole, btaOH, and NH4SCN in DMF in the presence of dioxygen allows isolation of the one‐dimensional coordination polymer [Mn3(btaO)2(NCS)4(DMF)8]n (3 ). X‐ray diffraction analysis reveals that the chain is best described as [Mn2(btaO)2(NCS)2(DMF)6] dimers linked by Mn(NCS)2(DMF)2 bridges. Molar magnetic susceptibility measurements of 3 in the 5−300 K range indicate weak antiferromagnetic coupling between the MnII atoms within the dinuclear subunits of the chain. (© Wiley‐VCH Verlag GmbH, 69451 Weinheim, Germany, 2002)
Abstract
The synthetic and structural chemistry of polynuclear manganese, cobalt, nickel and copper carboxylate complexes, stabilized by various forms of di-2-pyridyl ketone, is discussed. The structural diversity displayed by the described complexes stems from the ability of the doubly and singly deprotonated forms of the gem-diol form of di-2-pyridyl ketone, or the monoanion of the hemiacetal form of this ligand, to adopt a variety of coordination modes. The nuclearities of the clusters vary from four to fourteen. Perhaps the most aesthetically pleasing families are the "flywheel Cu 12 clusters, and the Co 9 and Ni 9 complexes in which the nine metal ions adopt a topology of two square pyramids sharing a common apex. A means of increasing the ground-state total spin value of a polynuclear 3d-metal cluster is also proposed. The approach is based on the replacement of hydroxo bridges, that most often propagate antiferromagnetic exchange interactions in clusters, by the end-on azido ligand, which is a well known ferromagnetic coupler. This approach involves "true" reactivity chemistry on pre-isolated clusters and the products are not undergone significant structural changes, except for the azido-for-hydroxo substitution, compared to the starting materials/clusters.
Abstract
Gridlock : A porous metal–organic framework with an interior tailored supramolecularly with organic groups is described (see structure; two grids have been differentiated using cyan and gray, while the yellow spheres represent included methyl groups). The approach relies on inverting the role of a secondary building unit (SBU) and an organic linker such that the organic linker serves as a node and the SBU serves as a linear bridge. The organic node used to construct the framework has been obtained by way of a template‐directed solid‐state organic synthesis.
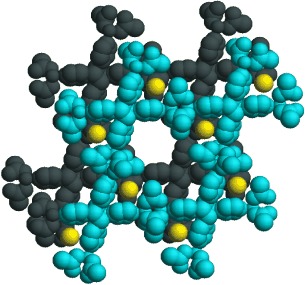
Abstract
Cocrystallization of 4-chlorostilbazole (4-Cl-sbz) with either resorcinol (res) or 4-ethylresorcinol (4-Et-res) yields three-component molecular assemblies, (res)·2(4-Cl-sbz) and (4-Et-res)·2(4-Cl-sbz), held together by two O−H···N hydrogen bonds. The olefins of each assembly conform to the distance criterion of Schmidt for [2+2] photoreaction. In the former, the olefins lie antiparallel, whereas in the latter, the olefins lie parallel. Both solids are photoactive, leading to the regiocontrolled solid-state synthesis of rctt-1,2-bis(4-pyridyl)-3,4-bis(4-chlorophenyl)cyclobutane (4-Cl-dpcb) in near or quantitative yield. The photoactivity of (res)·2(4-Cl-sbz) is attributed to the reactant undergoing a pedal-like change in conformation prior to cyclobutane formation. The ability to change the diol to achieve olefin alignment is expected to provide additional synthetic flexibility to this template approach.
2001 and older
Abstract
A systematic investigation of the [Cu2(O2CMe)4(H2O)2]/btd reaction system is described, where btd=2,1,3-benzothiadiazole. Reaction of [Cu2(O2CMe)4(H2O)2] with 5–8 equiv. of btd in both MeCN and MeOH yields [Cu2(O2CMe)4(btd)2] (1) in 40–50% yields. Treatment of [Cu2(O2CMe)4(H2O)2] with 1.4 or 0.7 equiv. of btd in MeOH leads to the precipitation of the polymeric compound {[Cu6(O2CMe)8(OMe)4(btd)2]} (2) in 82% yield. Reaction of 1 with two equivalents of [Cu2(O2CMe)4(H2O)2] in MeOH under reflux provides an additional route to 2. The structure of 1 consists of centrosymmetric dinuclear [Cu2(O2CMe)4(btd)2] molecules of the paddle-wheel cage type. The two CuII ions are bridged by four η1:η1:μ2 acetates, while a monodentate btd is at the apex of the square pyramid of each metal centre. The 2D structure of 2 consists of chains of tetranuclear, planar Cu4(OMe)4(O2CMe)4 repeating units running along the a axis, which are connected along the cell body diagonal via Cu2(O2CMe)4(btd)2 paddle-wheel dinuclear units. The btd molecules behave as bidentate bridging ligands. Within the tetranuclear units, each CuII ion is connected via two acetate bridges with one neighbouring CuII ion and via two methoxo bridges with the other neighbouring CuII ion; intertetranuclear linking is provided by two monoatomic acetate bridges. The results of solid state magnetic susceptibility studies are described for complex 2 in the temperature range 3–300 K. The results reveal antiferromagnetic exchange interactions between the CuII ions. The complicated structure of 2 does not permit an exact treatment for the determination of the various exchange interactions. However, an approximate 3-J magnetic model was constructed, resulting in an excellent fit. An orbital interpretation of the J-trend derived is also attempted.
[Cu2(O2CMe)4(H2O)2] reacts with an excess of the potentially bidentate bridging N,N′-donor 2,1,3-benzothiadiazole (btd) to give the dinuclear, paddle-wheel complex [Cu2(O2CMe)4(btd)2]. This complex has two free coordination sites and serves as a bridging “ligand” for the construction of a structurally and magnetically interesting 2D copper(II) coordination polymer.
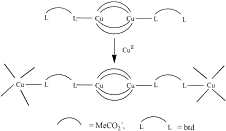
Abstract
Cocrystallization of 1,2-bis(4-pyridyl)ethane (4,4‘-bipyeth) with resorcinol (res), 4-chlororesorcinol (4-Cl-res), and 4,6-dichlororesorcinol (4,6-di-Cl-res) yields molecular solids (4,4‘-bipyeth)·(res) 1a, 2(4,4‘-bipyeth)·2(4-Cl-res) 1b, and 2(4,4‘-bipyeth)·2(4,6-di-Cl-res) 1c with components held together by O−H···N hydrogen bonds. In 1a the components form an infinite 1D polar array, whereas in 1b and 1c the components form 0D four-component complexes. Formation of the discrete assemblies is attributed to peripheral steric effects, which block the solid-state polymerization.
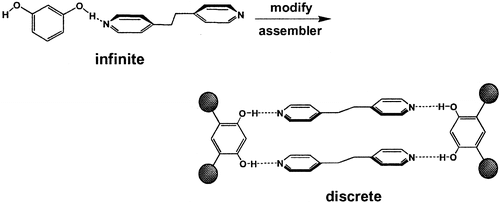
Abstract
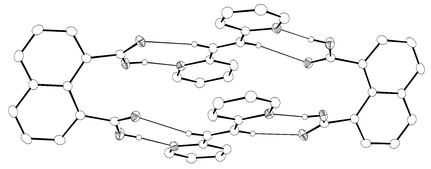
Abstract
The reaction of
Abstract
Cocrystallization of C-methylcalix[4]resorcinarene 1 with 4,4‘-bipyridine 2 in the presence of 4-bromobiphenyl (4-brbp) yields a molecular solid, of composition 3d·(4-brbp), [where 3d = 1·2(2)], in which 1 assembles with 2 to form a one-dimensional hydrogen-bonded polymer 3d that includes two copies of the biphenyl, within elliptical cavities of dimensions ∼ 11 × 7 × 6 Å, as guests. The framework 3d represents a third architectural isomer of three previously reported supramolecular isomers of the parent material 3.
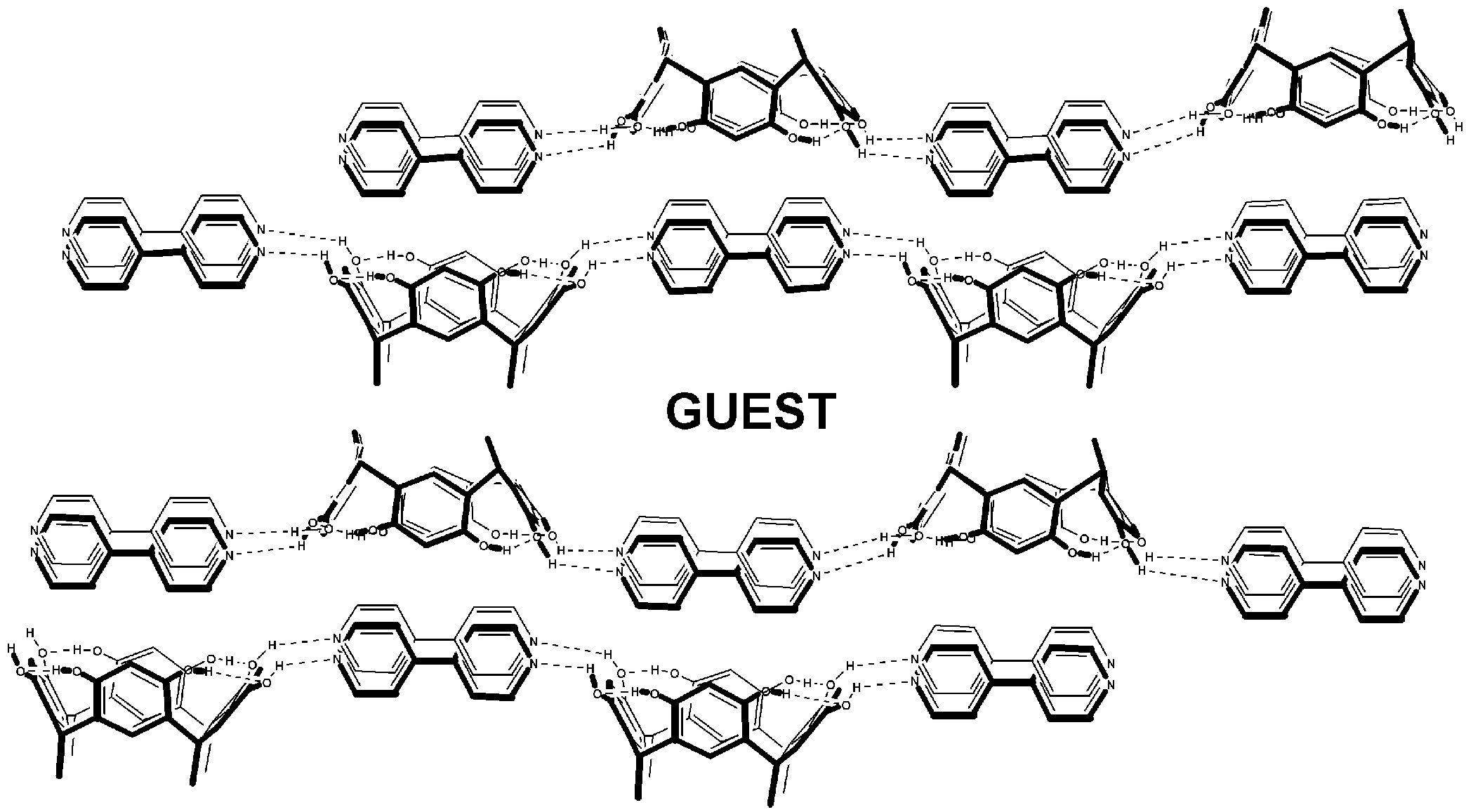
Abstract
The reactions of CoX2 (X=Br, Cl) with the planar, bidentate bridging ligand 2,1,3-benzothiadiazole (btd) in Me2CO led to the 2D coordination polymers {[CoBr2(btd)]}n (1) and {[CoCl2(btd)]}n (2). The structure of 1 was determined by single-crystal X-ray crystallography. Complex 1 (C6H4Br2CoN2S, monoclinic, P21/m, a=3.742(3), b=13.075(9), c=9.092(6) Å, β=90.09(2)°, Z=2, θ–2θ, R1=0.0298, wR2=0.0793) consists of {[Co(μ-Br)2]}n linear chains running along the a axis linked via μ-btd ligands along the b axis. The columns of stacked btd molecules present in the crystal structure of the free ligand are maintained in the lattice of 1. XRD data revealed that 2 is isostructural with 1. The magnetic properties of both complexes can be explained by the presence of a very weak ferromagnetic intrachain Co···Co exchange interaction through the (μ-X)2 bridges and a moderate antiferromagnetic Co···Co interaction through the μ-btd bridges. The new complexes were also characterized by EPR, IR, and UV/VIS spectra, and all data are discussed in terms of the nature of bonding and known structures.
Abstract
The reactions of M(O2CCH3)2·4H2O (M= Mn, Co) with di‐2‐pyridyl ketone, (py)2CO, and NaN3 in CH3OH or CH3OH/H2O allow isolation of the tetranuclear compounds [Mn4(μ1,1‐N3)2(N3)2{(py)2C(OH)O}2{(py)2C(OCH3)O}2] (1) and [Co4(μ1,1‐N3)2(N3)2{(py)2C(OH)O}2{(py)2C(OCH3)O}2]·2H2O (2). X‐ray diffraction analysis reveals defective double‐cubane tetrameric entities in which the MnII or CoII atoms are linked by μ1,1‐N3 bridges and two kinds of O‐bridges. The molar magnetic susceptibility measurements of 1 and 2 in the 2−300 K range indicate weak antiferromagnetic coupling for 1 and clear bulk ferromagnetic coupling for 2 .
Abstract
μ4 end‐on coordination (through the N atom) of the pseudohalogeno ligands X−=N3− and NCO− has been observed in the isostructural nonanuclear CoII cages with the general formula [Co9{(2‐C5H4N)2CO2}4(O2CMe)8X2] (see picture); this mode is imposed by the trapping of anions X− into cavities formed inside the cage. The change of X− modulates the magnetic behavior of the cage from antiferromagnetic coupling to a ferrimagnetic high‐spin ground state.
Abstract
This work is devoted to structural and vibrational studies of N-alkyloxamates and their complexes. The structures of MeHNCOCOOK and [Cu(EtHNCOCOO)2] have been determined by single-crystal X-ray crystallography. The potassium salt crystallizes in the monoclinic space group P21/n and the copper(II) complex in the triclinic space group The lattice constants are a=3.882(2), b=10.175(1), c=13.004(1), β=97.04(1)° for MeHNCOCOOK and a=5.013(1), b=6.996(1), α=73.54(1), β=85.66(1), γ=71.22(1)° for [Cu(EtHNCOCOO)2]. The salt has a trans secondary amide group and the K− ion is surrounded by seven O atoms belonging to six different N-methyloxamate(-1) moieties. The ligand EtHNCOCOO− behaves as a bidentate chelate in the trans square planar copper(II) complex with ligated atoms being the amide and one of the carboxylate oxygens. The copper(II) complex was characterized by its room-temperature magnetic susceptibility and solid-state d–d spectrum. A detailed comparison of the vibrational spectra (FT-IR, FT-Raman) of [Cu(EtHNCOCOO)2] with the corresponding potassium salt, EtHNCOCOOK, is presented. The vibrational data are discussed in terms of the nature of bonding and the known structure of the copper(II) complex. Quantum-chemical calculations were performed to obtain the equilibrium geometries and torsional energies for an isolated oxamate(-1) ion (H2NCOCOO−) and its hypothetical square planar zinc(II) complex.
Abstract
The synthesis, structure, vibrational analysis and a comparative vibrational study in function of the structure and the metal–ligand bond strength are discussed for a series of Mn(II)–urea–halogenide complexes. Complex [MnCl2U] has been prepared and studied for the first time. The single-crystal X-ray structure of the 1:6-bromide complex reveals the presence of octahedral [MnU6]2+ ions and bromide counterions.
Abstract
Employing a variety of solvents and molar ratios, the reactions of Mn(NO3)2·6H2O with N,N′-dimethylurea (DMU) afforded the adduct [Mn(NO3)2(DMU)3] (1). X-ray analysis shows that the high-spin complex has a pentagonal bipyramidal geometry with two DMU oxygen atoms in axial positions and with two types of monodentate O-bonding for the DMU molecules, one of them being very unusual. The spectroscopic properties of the prepared complex are also discussed.
