Design, synthesis and study of phospholipase A2 inhibitors - Novel compounds for the treatment of inflammatory and autoimmune diseases
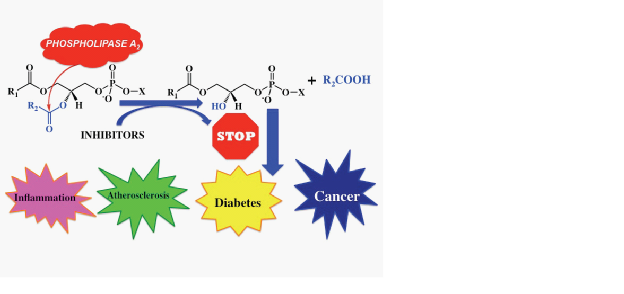
Phospholipase A2 (PLA2) enzymes catalyze the hydrolysis of the ester bond at the sn-2 position of glycerophospholipids releasing free fatty acids, including arachidonic acid and lysophospholipids. Arachidonic acid may be metabolized to various bioactive eicosanoids, while lysophospholipids may produce PAF. All these products are precursor signaling molecules that are involved in inflammation and pain. The three predominant types of phospholipase A2 (PLA2) found in human tissues are the cytosolic (such as the GIVA cPLA2), the secreted (such as the GIIA and GV sPLA2), and the calcium-independent (such as the GVIA iPLA2) enzymes. Although these enzymes display common enzymatic activities, each PLA2 plays distinct roles in the molecular signaling and metabolism of phospholipids. The development of selective inhibitors for individual PLA2 enzymes is of great importance in order to target PLA2-specific signaling pathways and develop new agents for the treatment of pain, inflammation and diabetes.
“Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention”. Dennis, E. A.; Cao, J.; Hsu, Y. H.; Magrioti, V.; Kokotos, G. Chem. Rev. 2011, 111, 6130–6185.
“Phospholipase A2 inhibitors for the treatment of inflammatory diseases: a patent review (2010 – present)”. Magrioti, V.; Kokotos, G. Expert Opin. Ther. Pat. 2013, 23, 333–344.
“Synthetic and natural inhibitors of phospholipases A2: Their importance for understanding and treatment of neurological disorders”. Ong, W.-Y.; Farooqui, T.; Kokotos, G.; Farooqui, A. A. ACS Chem. Neurosci. 2015, 6, 814-831.
“Inhibitors of phospholipase A2 and their therapeutic potentials: an update on patents (2012-2016)” M. G. Kokotou, D. Limnios, A. Nikolaou, A. Psarra, G. Kokotos, Exp. Opin. Ther. Patents 2016, Oct 8. [Epub ahead of print].
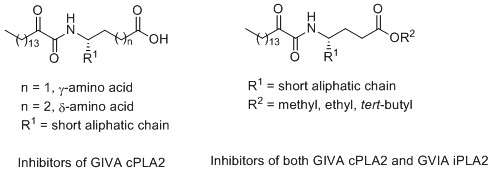
2-Oxoamide inhibitors
2-Oxoamides based on γ- or δ-amino acids containing a free carboxylic group are potent and selective inhibitors of GIVA cPLA2 presenting in vivo anti-inflammatory and analgesic activity. The 2-oxoamide ethyl ester derivative AX048, which in vitro inhibits both GIVA cPLA2and GVIA iPLA2, presents a potent analgesic effect.
“Structure-activity relationship of 2-oxoamide inhibition of group IVA cytosolic phospholipase A2 and group V secreted phopholipase A2”. Six, D. A.; Barbayianni, E.; Loukas, V.; Constantinou-Kokotou, V.; Hadjipavlou-Litina, D.; Stephens, D.; Wong, A. C.; Magrioti, V.; Moutevelis-Minakakis, P.; Baker, S.; Dennis, E. A.; Kokotos, G. J. Med. Chem. 2007, 50, 4222–4235.
“Systemic and intrathecal effects of a novel series of phospholipase A2 inhibitors on hyperalgesia and spinal prostaglandin E2 release”. Yaksh, T. L.; Kokotos, G.; Svensson, C. I.; Stephens, D.; Kokotos, C. G. Fitzsimmons, B.; Hadjipavlou-Litina, D.; Hua, X. Y.; Dennis, E. A. J. Pharmacol. Exper. Ther. 2006, 316, 466-475.
“Differential inhibition of group IVA and group VIA phospholipases A2 by 2-oxoamides”. Stephens, D.; Barbayianni, E.; Constantinou-Kokotou, V.; Peristeraki, A.; Six, D. A.; Cooper, J.; Harkewicz, R.; Deems, R. A.; Dennis, E. A.; Kokotos, G. J. Med. Chem. 2006, 49, 2821–2828.
“Inhibition of group IVA cytosolic phospholipase A2 by novel 2-oxoamides in vitro, in cells and in vivo”. Kokotos, G.; Six, D. A.; Loukas, V.; Smith, T.; Constantinou-Kokotou, V.; Hadjipavlou-Litina, D.; Kotsovolou, S.; Chiou, A.; Beltzner, C. C.; Dennis, E. A. J. Med. Chem. 2004, 47, 3615–3628.
“Novel 2-oxoamide inhibitors of human group IVA phospholipase A2”. Kokotos, G.; Kotsovolou, S.; Six, D. A.; Constantinou-Kokotou, V.; Beltzner, C. C.; Dennis, E. A. J. Med. Chem. 2002, 45, 2891–2893.
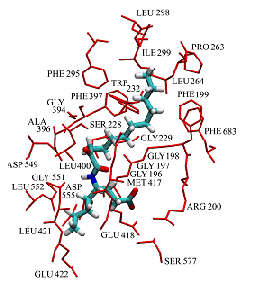
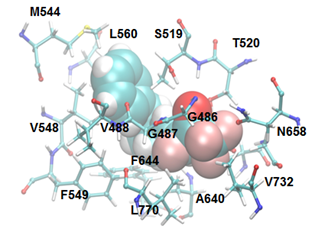
Study of the enzyme-inhibitor interactions
The location of an oxoamide inhibitor and a fluoroketone inhibitor within the active site of GIVA cPLA2 and GVIA iPLA2, respectively, has been determined by a combination of deuterium exchange mass spectrometry with molecular dynamics.
“Location of inhibitors bound to group IVA phospholipase A2 determined by molecular dynamics and deuterium exchange mass spectrometry”. Burke, J. E.; Babakhani, A.; Gorfe, A. A.; Kokotos, G.; Li, S.; Woods, V. L., Jr.; McCammon, J. A.; Dennis, E. A. J. Am. Chem. Soc. 2009, 131, 8083–8091.
“Fluoroketone inhibition of Ca2+- independent phospholipase A2 through binding pocket association defined by hydrogen/deuterium exchange and molecular dynamics” Y. H. Hsu, D. Bucher, J. Cao, S. Li, S. W. Yang, G. Kokotos, V. L. Woods, J. A. McCammon, E. A. Dennis J. Am. Chem. Soc. 2013, 135, 1330-1337.
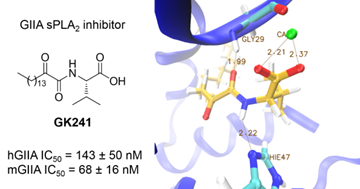
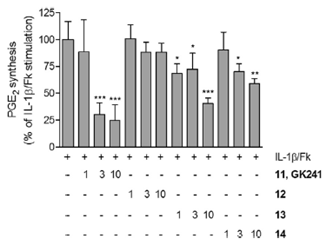
2-Oxoamides based on α-amino acids are selective inhibitors of secreted GIIA sPLA2. We have demonstrated that sPLA2 inhibitors suppress the release of PGE2 in renal mesangial cells.
“Development of a potent 2-oxoamide inhibitor of secreted phospholipase A2 guided by molecular docking calculations and molecular dynamics simulations”.Vasilakaki, S.; Barbayianni, E.; Leonis, G.; Papadopoulos, M. G.; Mavromoustakos, T.; Gelb, M. H.; Kokotos, G. Bioorg. Med. Chem. 2016, 24, 1683-1695.
“Inhibitors of secreted phospholipase A2 suppress the release of PGE2 in renal mesangial cells” S. Vasilakaki, E. Barbayianni, V. Magrioti, O. Pastukhov, V. Constantinou-Kokotou, A. Huwiler, G. Kokotos Bioorg. Med. Chem. 2016, 24, 3029-3034.
Fluoroketones
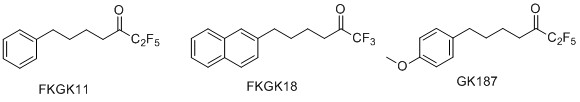
We have developed polyfluoroketones as a novel class of calcium-independent PLA2 (GVIA iPLA2) inhibitors. FKGK11, FKGK18 and GK187 are potent and selective inhibitors of GVIA iPLA2. We have demonstrated that selective inhibition of iPLA2 can ameliorate the progression of Experimental Autoimmune Enkephalomyelitis. Such inhibitors of iPLA2 might therefore be good candidates as new agents for the treatment of multiple sclerosis.
“Development of potent and selective inhibitors for Group VIA calcium-independent phospholipase A2 quided by molecular dynamics and structure-activity relationships” V. D. Mouchlis, D. Limnios, M. G. Kokotou, E. Barbayianni, G. Kokotos, J. A. McCammon, E. A. Dennis, J. Med. Chem. 2016, 59, 4403-4414.
“Inhibition of Ca2+-independent phospholipase A2 (iPLA2β) ameliorates islet infiltration incidence of diabetes in NOD mice.” Bone R. N, Gai Y, Magrioti V, Kokotou M. G, Ali T, Lei X, Tse H. M, Kokotos G, Ramanadham S. Diabetes 2015, 64, 541-554.
“New potent and selective polyfluoroalkyl ketone inhibitors of GVIA calcium-independent phospholipase A2”. Magrioti, V.; Nikolaou, A.; Smyrniotou, A.; Shah, I.; Constantinou-Kokotou, V.; Dennis, E. A.; Kokotos, G. Bioorg. Med. Chem. 2013, 21, 5823-5829.
“Potent and selective fluoroketone inhibitors of group VIA calcium-independent phospholipase A2”. Kokotos, G.; Hsu, Y.-H.; Burke, J. E.; Baskakis, C.; Kokotos, C. G.; Magrioti V.; Dennis, E. A. J. Med. Chem. 2010, 53, 3602-3610.
“Differing roles for members of the phospholipase A2 superfamily in experimental autoimmune encephalomyelitis”. Kalyvas, A.; Baskakis, C.; Magrioti, V.; Constantinou-Kokotou, V.; Stephens, D.; Lopez-Vales, R.; Lu, J.-Q.; Yong, V.; Dennis, E. A.; Kokotos, G.; David, S. Brain 2009.
“Synthesis of polyfluoro ketones for selective inhibition of human phospholipase A2 enzymes”. Baskakis, C.; Magrioti, V.; Cotton, N.; Stephens, D.; Constantinou-Kokotou, V.; Dennis, E.A.; Kokotos, G. J. Med. Chem. 2008, 51, 8027-8037.
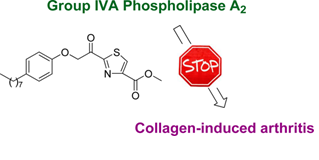
Thiazolyl ketones
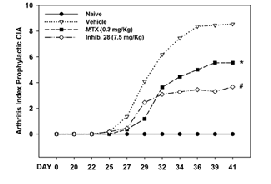
“Inhibition of group IVA cytosolic phospholipase A2 by thiazolyl ketones in vitro, ex vivo, and in vivo”. Kokotos, G.; Feuerherm, A. J.; Barbayianni, E.; Shah, I.; Sæther, M; Magrioti, V.; Nguyen, T.; Constantinou-Kokotou, V.; Dennis, E. A.; Johansen, B. J. Med. Chem. 2014, 57, 7523–7535.
Design, synthesis and study of autotaxin inhibitors
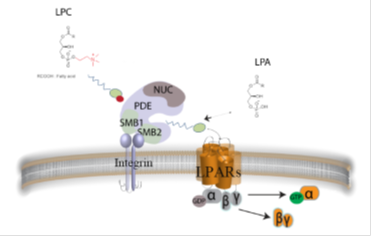
Autotaxin (ATX) possesses lysophospholipase D activity, catalyzing the hydrolysis of lysophosphatidylcholine into lysophosphatidic acid (LPA), and it is considered the major LPA-producing enzyme in the circulation. LPA is a bioactive phospholipid with diverse functions in almost every mammalian cell type, which exerts its action through binding to specific G protein-coupled receptors and stimulates various cellular functions, including migration, proliferation and survival. As a consequence, both ATX and LPA have attracted the interest of researchers, in an effort to understand their roles in physiology and pathophysiology. We have developed novel inhibitors of ATX, in an effort to develop novel medicines to treat inflammatory diseases and cancer.
“Autotaxin, a secreted lysophospholipase D, as a promising therapeutic target in chronic inflammation and cancer” E. Barbayanni, E. Kaffe, V. Aidinis, G. Kokotos Progr. Lipid Res. 2015, 58, 76-96
“Autotaxin inhibitors: A patent review” E. Barbayianni, V. Magrioti, P. Moutevelis-Minakakis, G. Kokotos Expert Opin. Ther. Patents 2013, 23, 1123-1132.