Inhibitors of the Enzymes of the Endocannabinoid System
Cannabis sativa is one of the oldest plants cultivated by the human race, not for food, but for its use for medical and recreational purposes. The Chinese seem to have used it both as medicine and as textile for its fibers as early as in 4000 B.C. In India, the medical and religious use of Cannabis begun around 1000 B.C., while Herodotus described a Scythian funeral ceremony in 450 B.C. where cannabis seeds burned and the participants inhaled its vapors as part of the ritual. It took many centuries to discover the main psychoactive compound of this plant. In 1964, Gaoni and Mechoulam isolated and elucidated Ä9-tetrahydrocannabinol (Ä9-THC) as the active compound with the well known psychotropic activities. Early in the 1990s, the two specific receptors for Ä9-THC were discovered. CB1 receptor is one of the most abundant neuromodulatory receptors in the brain and is expressed at high levels in the hippocampus, cortex, cerebellum, and basal ganglia, a fact that corresponds to the phychoactive properties of cannabis use. On the other hand, CB2 receptor is mainly present in the immune system and is supposed to be involved in the regulation of immune responses and/or inflammatory reactions. After the discovery of the CB receptors, researchers started looking for endogenous ligands for these receptors, and since Ä9-THC is a lipophilic compound, they were looking for lipophilic compounds abundant in the brain. The most important endocannabinoids that bind to CB1 and CB2 receptors are anandamide (N-Arachidonoylethanolamine) and 2-arachidonoylglycerol (2-AG), with 2-AG being the most abundant one. They both induce the cannabimimetic activities in vitro and in vivo. The two enzymes that hydrolyse these endocannabinoids are fatty acid amide hydrolase (FAAH) for anandamide and monoacylglycerol lipase (MGL) for 2-AG.
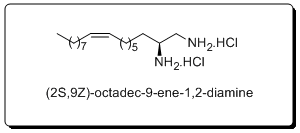
The endocannabinoid system is an interesting and evolving target for the development of new medicines. In our research, we focus on selective inhibitors of MGL and we have identified (2S,9Z)-octadec-9-ene-1,2-diamine as a potent selective inhibitor of MGL when compared to FAAH. This diamine also exhibited in vivo anti-inflammatory and analgesic activities, a fact that suggests that selective MGL inhibitors may be used for the treatment of inflammatory pain.
The endocannabinoid system is an interesting and evolving target for the development of new medicines. In our research, we focus on selective inhibitors of MGL and we have identified (2S,9Z)-octadec-9-ene-1,2-diamine as a potent selective inhibitor of MGL when compared to FAAH. This diamine also exhibited in vivo anti-inflammatory and analgesic activities, a fact that suggests that selective MGL inhibitors may be used for the treatment of inflammatory pain.
References
1. V. Magrioti, G. Naxakis, D. Hadjipavlou-Litina, A. Makriyannis, G. Kokotos, Bioorg. Med. Chem. Lett. 2008, 18, 5424-5427.
1. V. Magrioti, G. Naxakis, D. Hadjipavlou-Litina, A. Makriyannis, G. Kokotos, Bioorg. Med. Chem. Lett. 2008, 18, 5424-5427.
