Publications
Table of Contents (TOC)
Anion Exchange in Inorganic Perovskite Nanocrystal Polymer Composites
Chemical Science 2018, 9(42), 8121–8126. doi: 10.1039/c8sc02830c
Abstract
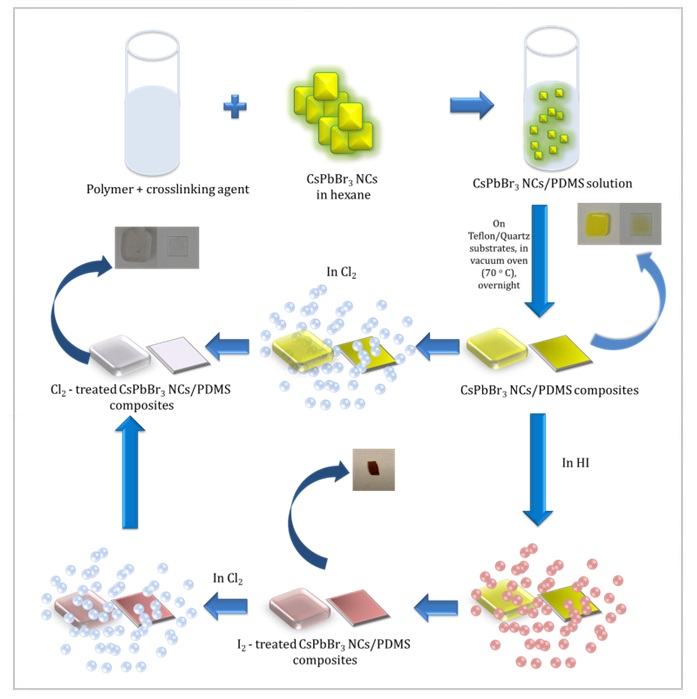
We demonstrate a facile, low-cost, and room-temperature method of anion exchange in cesium lead bromide nanocrystals (CsPbBr₃ NCs), embedded into a polymer matrix. The anion exchange occurs upon exposure of the solid CsPbBr₃ NCs/PDMS nanocomposite to a controlled anion precursor gas atmosphere. The rate and extent of the anion exchange reaction can be controlled via variation of either the exposure time or the relative concentration of the anion precursor gas. Post-synthesis chemical transformation of perovskite nanocrystal polymer composites is not readily achievable using conventional methods of anion exchange, which renders the gas-assisted strategy extremely useful. We envisage that this work will enable the development of solid-state perovskite NC optoelectronic devices.
For citation:
Sygletou, M. E.; Kyriazi, M. E.; Kanaras, A. G.; Stratakis, E.
"Anion Exchange in Inorganic Perovskite Nanocrystal Polymer Composites"
Chem. Sci. 2018, 9(42), 8121–8126. doi: 10.1039/c8sc02830c