Current research
Structure-function relationships in selected transporters
Very little is known on transporter
structure-function relationships due to the difficulties in obtaining
X-ray diffraction-quality crystals and in applying NMR methods for the study
of polytopic membrane proteins. Furthermore, the few currently known
structures come exclusively from bacteria and archea, and in the majority of
cases are not associated with functional studies. An alternative approach to
understand structure-function relationships of transporters and channels is
through functional analysis of mutated or molecularly modified versions of
these proteins in easily manipulated systems. The magnificent work of R.
Kaback and his group with the LacY lactose permease of Escherichia coli
constitutes a unique paradigm in biology. In this case, solely through the
analysis of mutations, biochemical and biophysical approaches and the
development of Cys-scanning mutagenesis, a model was proposed on how a
transporter works, even before the eventual crystallization of LacY (Guan L,
Kaback HR. 2006. Annu Rev Biophys
Biomol Struct, 35:67-91).
The contribution of our lab in this direction concerns the use of classical
and reverse genetics, direct biochemical transport assays,
in vivo fluorescent microscopy
and kinetic modeling to understand how several purine, pyrimidine or amino
acid transporters work (for reviews see Diallinas, 2008; Pantazopoulou and
Diallinas, 2008; Gournas et al, 2008; Diallinas & Gournas 2008).
By far our favorite molecular is the
UapA uric acid-xanthine/H+ symporter. UapA is the prototype
and founding member of
an important and
ubiquitous transporter family, called Nucleobase Ascorbate Transporters
(NAT). At present, the function and specificity of nearly 20 NAT proteins
from bacteria, fungi, plants and mammals is known. Seven of these NATs (six
fungal and one plant) have been characterized and studied in our lab. All
non-mammalian homologues of known function are specific for nucleobases,
namely xanthine, uric acid or uracil. The mammalian NATs transport either
L-ascorbic acid (SVCT1 and SVCT2) or nucleobases, namely uracil, xanthine or
hypoxanthine (rSNBT17).
In the last 15 years, hundreds of UapA mutations, obtained by classical or
reverse genetic approaches, as well as chimeric constructs, have been
analyzed at the molecular, cellular and functional level, giving rise to
unprecedented knowledge of the molecular elements underlying the function of
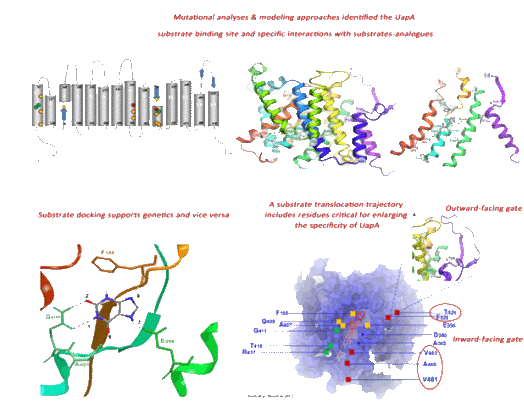
this eukaryotic carrier. Based on our results, we have proposed models on
how the UapA recognizes and translocates its substrates, and provided data
on which amino residues are involved in substrate selection, binding and
transport, and on which amino acid residues are key elements for protein
stability and trafficking to the plasma membrane. In fact, it is through our
studies that channel-like gating
has been proposed to exist and be critical in determining UapA transporter
specificity. This is an entirely novel concept that breaks the dogmatic
distinction of transporters and channels. Supporting evidence for this idea
has recently come form direct structural studies on other transporters.
At present, we try to understand in more detail how the binding and release
of substrates leads to the opening and closing of the substrate
translocation trajectory, how the gates synergize with the major substrate
binding site, how our findings are related to the generally accepted model
of outward- and inward-facing alternating transporter conformers
(rocker-switch mechanism), and how ions drive solute symport. We also ask
whether we can engineer new UapA versions transporting potential antifungal
drugs and how domains external to the substrate biding site act as filters
or gates affecting UapA substrate specificity.
Similar approaches of those used to study UapA, are currently followed to
study other NAT members (AzgA, UapD) and selected members of an evolutionary
and functionally district purine-pyrimidines transporter family (NCS1
transporters).
Membrane trafficking and endocytosis of transporters
Eukaryotic polytopic membrane proteins, such as transporters and channels,
or receptors, are co-translationally inserted into the ER membrane through
the action of the so-called translocase complex. This mechanistic step seems
to be driven by the energy of polypeptide synthesis in the ribosome and
several sequence-independent, amphipathicity-dependent, cis-acting elements
on the protein cargoes. Once a membrane protein is properly folded within
the ER membrane, an important check-point by itself, it then follows a
vesicular or tubular trafficking pathway, initially towards the Golgi, and
subsequently towards the endosomal pathway, the vacuole or the plasma
membrane. This long sub-cellular journey (exocytosis) is dynamically
controlled in response to multiple and overlapping developmental and
physiological signals, rather than being a default process. Such signals not
only promote or arrest exocytosis of a protein towards its target membrane,
but can also promote its rapid endocytosis from the plasma membrane, which
can lead to degradation in the vacuole or recycling back to the cell
surface. The sum of complex processes underlying membrane protein exocytosis
and endocytosis is called membrane protein trafficking. In this process,
both cis-acting elements on the
cargo proteins and trans-acting factors need to be orchestrated in a
sequential and flexible manner to achieve proper trafficking. Moreover, it
has been recently shown that the lipid composition of membranes, which by
itself is dynamically controlled in response to various signals, plays a
pivotal role in protein trafficking. The dynamic control of the trafficking
of proteins such as transporters, channels and receptors, proteins, which
play essential roles in the uptake or efflux of metabolites and drugs or the
transmission of molecular signals, constitutes a rapid, flexible and very
efficient regulatory mechanism critical for cell homeostasis and for the
communication of cells with their environment.
In the yeast S. cerevisiae
several plasma membrane transporters have been used extensively in order to
understand membrane cargo trafficking, sorting and endocytosis. In most
cases, transporter trafficking is controlled in response to substrate
availability, general nutrient supply conditions and/or stresses.
Strikingly, the mechanisms controlling transporter trafficking are
essentially conserved from fungi to mammals. In fact, studies on the
intracellular trafficking of yeast permeases have contributed to revealing
the central role played by the small protein ubiquitin (Ub), a sorting
signal of eukaryotic membrane proteins. In yeasts and
A. nidulans the covalent
attachment of Ub on cargoes depends on Rsp5/HulA, a Ub ligase of the Nedd4
HECT family. Recent studies suggested a general model in which different
Rsp5/HulA adaptor proteins recognize different transporters, or the same
transporter in response to different stimuli. Finally, lipid rafts, formed
by the lateral association of sphingolipids and cholesterol (mammals) or
ergosterol (fungi) in the external membrane leaflet have been implicated in
transporter traffic and cell signaling in mammalian cells and yeast.
Several genetic and molecular tools for specific sub-cellular
organelles/compartments have also been developed for A. nidulans
(mostly in the lab of
M.A. Penalva) and several
transporters of purines, pyrimidines and amino acids, belonging to
evolutionary discrete families, have been used as protein cargoes to study
endocytosis in response to a shift in nitrogen source or excess substrate
(our lab). As a consequence, important aspects of trafficking mechanisms
have been revealed in this model fungus. The primary contribution of our lab
in this direction is the identification of two distinct
mechanisms controlling transporter down-regulation by endocytic
internalization. The first occurs in response to a shift from poor to rich
nitrogen media (ammonium ions) and the second in response to substrate
excess (Valdez-Taubas et al. 2000; Pantazopoulou et al. 2007; Gournas et al.
2010). In the case of the UapA transporter, both mechanisms are dependent on
HulA-dependent ubiquitination of a single
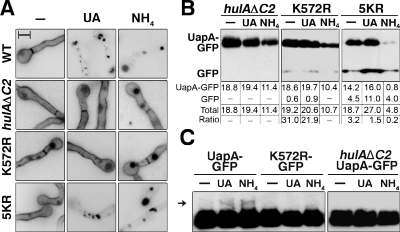
A, UapA-GFP endocytosis elicited by NH4 and
substrates (UA) showing the requirement of HulA & K572R.
B-C, UapA-GFP turnover and
ubiquitination by NH4 or UA
-
How mutations resulting in transporter intrinsic instability lead to enhanced endocytosis and vacuolar degradation?
-
Which cargo cis-acting elements are involved in the "molecular conversation" with the trafficking machinery?
-
Which are the pathways followed, after exit from the Golgi, of a newly made transporter under different physiological conditions or in specific mutants?
-
What type of ubiquitination (mono-, multiple-, poly-, K63 chains) is involved and at which cellular compartments this takes place during transporter trafficking, in response to different physiological conditions or in mutants affected in trafficking?
-
What is the role of lipid composition in transporter trafficking, endocytosis and vacuolar degradation?
-
Do different transporters follow similar pathways or use similar mechanisms for trafficking? Most of our initial studies employ as a model cargo the uric acid permease UapA. Using the originally obtained knowledge, subsequent studies will investigate the trafficking of several other transporters (UapC, FurD, AzgA, FcyB, CntA, PrnB, AgtA) readily available in our lab.